Delayed administration of N-acetylcysteine blunts recovery after an acetaminophen overdose unlike 4-methylpyrazole
- PMID: 34420083
- PMCID: PMC8448936
- DOI: 10.1007/s00204-021-03142-9
Delayed administration of N-acetylcysteine blunts recovery after an acetaminophen overdose unlike 4-methylpyrazole
Abstract
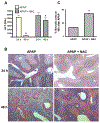
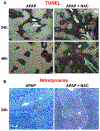
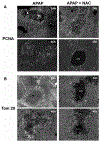
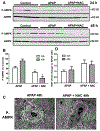
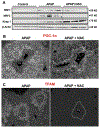
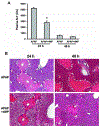
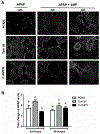
N-acetylcysteine (NAC) is the only clinically approved antidote against acetaminophen (APAP) hepatotoxicity. Despite its efficacy in patients treated early after APAP overdose, NAC has been implicated in impairing liver recovery in mice. More recently, 4-methylpyrazole (4MP, Fomepizole) emerged as a potential antidote in the mouse APAP hepatotoxicity model. The objective of this manuscript was to verify the detrimental effect of NAC and its potential mechanism and assess whether 4MP has the same liability. C57BL/6J mice were treated with 300 mg/kg APAP; 9 h after APAP and every 12 h after that, the animals received either 100 mg/kg NAC or 184.5 mg/kg 4MP. At 24 or 48 h after APAP, parameters of liver injury, mitochondrial biogenesis and cell proliferation were evaluated. Delayed NAC treatment had no effect on APAP-induced liver injury at 24 h but reduced the decline of plasma ALT activities and prevented the shrinkage of the areas of necrosis at 48 h. This effect correlated with down-regulation of key activators of mitochondrial biogenesis (AMPK, PGC-1α, Nrf1/2, TFAM) and reduced expression of Tom 20 (mitochondrial mass) and PCNA (cell proliferation). In contrast, 4MP attenuated liver injury at 24 h and promoted recovery at 48 h, which correlated with enhanced mitochondrial biogenesis and hepatocyte proliferation. In human hepatocytes, 4MP demonstrated higher efficacy in preventing cell death compared to NAC when treated at 18 h after APAP. Thus, due to the wider treatment window and lack of detrimental effects on recovery, it appears that at least in preclinical models, 4MP is superior to NAC as an antidote against APAP overdose.
Keywords: Acetaminophen; Drug hepatotoxicity; Fomepizole; Mitochondrial biogenesis; N-acetylcysteine; Regeneration.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures

References
-
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H (2000) Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci 58:109–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

