Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction
- PMID: 34420373
- PMCID: PMC8462445
- DOI: 10.1161/CIRCULATIONAHA.121.056177
Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction
Abstract
Background: Inflammation is a key factor of myocardial damage in reperfused ST-segment-elevation myocardial infarction. We hypothesized that colchicine, a potent anti-inflammatory agent, may reduce infarct size (IS) and left ventricular (LV) remodeling at the acute phase of ST-segment-elevation myocardial infarction.
Methods: In this double-blind multicenter trial, we randomly assigned patients admitted for a first episode of ST-segment-elevation myocardial infarction referred for primary percutaneous coronary intervention to receive oral colchicine (2-mg loading dose followed by 0.5 mg twice a day) or matching placebo from admission to day 5. The primary efficacy outcome was IS determined by cardiac magnetic resonance imaging at 5 days. The relative LV end-diastolic volume change at 3 months and IS at 3 months assessed by cardiac magnetic resonance imaging were among the secondary outcomes.
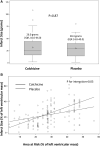
Results: We enrolled 192 patients, 101 in the colchicine group and 91 in the control group. At 5 days, the gadolinium enhancement-defined IS did not differ between the colchicine and placebo groups with a mean of 26 interquartile range (IQR) [16-44] versus 28.4 IQR [14-40] g of LV mass, respectively (P=0.87). At 3 months follow-up, there was no significant difference in LV remodeling between the colchicine and placebo groups with a +2.4% (IQR, -8.3% to 11.1%) versus -1.1% (IQR, -8.0% to 9.9%) change in LV end-diastolic volume (P=0.49). Infarct size at 3 months was also not significantly different between the colchicine and placebo groups (17 IQR [10-28] versus 18 IQR [10-27] g of LV mass, respectively; P=0.92). The incidence of gastrointestinal adverse events during the treatment period was greater with colchicine than with placebo (34% versus 11%, respectively; P=0.0002).
Conclusions: In this randomized, placebo-controlled trial, oral administration of high-dose colchicine at the time of reperfusion and for 5 days did not reduce IS assessed by cardiac magnetic resonance imaging. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03156816.
Keywords: clinical trial; colchicine; heart injuries; inflammation; myocardial infarction; thrombosis; ventricular remodeling.
Figures

References
-
- Anzai T. Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J. 2013;77:580–587. doi: 10.1253/circj.cj-13-0013 - PubMed
-
- Bochaton T, Lassus J, Paccalet A, Derimay F, Rioufol G, Prieur C, Bonnefoy-Cudraz E, Crola Da Silva C, Bernelin H, Amaz C, et al. . Association of myocardial hemorrhage and persistent microvascular obstruction with circulating inflammatory biomarkers in STEMI patients. PLoS One. 2021;16:e0245684. doi: 10.1371/journal.pone.0245684 - PMC - PubMed
-
- Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. doi: 10.1016/j.jacc.2014.01.014 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

