Novel Mechanism for an Old Drug: Phenazopyridine is a Kinase Inhibitor Affecting Autophagy and Cellular Differentiation
- PMID: 34421588
- PMCID: PMC8371461
- DOI: 10.3389/fphar.2021.664608
Novel Mechanism for an Old Drug: Phenazopyridine is a Kinase Inhibitor Affecting Autophagy and Cellular Differentiation
Abstract
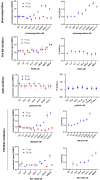
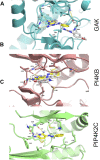
Phenazopyridine is a widely used drug against urinary tract pain. The compound has also been shown to enhance neural differentiation of pluripotent stem cells. However, its mechanism of action is not understood. Based on its chemical structure, we hypothesized that phenazopyridine could be a kinase inhibitor. Phenazopyridine was investigated in the following experimental systems: 1) activity of kinases in pluripotent stem cells; 2) binding to recombinant kinases, and 3) functional impact on pluripotent stem cells. Upon addition to pluripotent stem cells, phenazopyridine induced changes in kinase activities, particularly involving Mitogen-Activated Protein Kinases, Cyclin-Dependent Kinases, and AKT pathway kinases. To identify the primary targets of phenazopyridine, we screened its interactions with 401 human kinases. Dose-inhibition curves showed that three of these kinases interacted with phenazopyridine with sub-micromolar binding affinities: cyclin-G-associated kinase, and the two phosphatidylinositol kinases PI4KB and PIP4K2C, the latter being known for participating in pain induction. Docking revealed that phenazopyridine forms strong H-bonds with the hinge region of the ATP-binding pocket of these kinases. As previous studies suggested increased autophagy upon inhibition of the phosphatidyl-inositol/AKT pathway, we also investigated the impact of phenazopyridine on this pathway and found an upregulation. In conclusion, our study demonstrates for the first time that phenazopyridine is a kinase inhibitor, impacting notably phosphatidylinositol kinases involved in nociception.
Keywords: autophagy; differentiation; kinase; phenazopyridine; phosphatidylinositol kinase.
Copyright © 2021 Preynat-Seauve, Nguyen, Westermaier, Héritier, Tardy, Cambet, Feyeux, Caillon, Scapozza and Krause.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

