The Phospholipid N-Methyltransferase and Phosphatidylcholine Synthase Pathways and the ChoXWV Choline Uptake System Involved in Phosphatidylcholine Synthesis Are Widely Conserved in Most, but Not All Brucella Species
- PMID: 34421831
- PMCID: PMC8371380
- DOI: 10.3389/fmicb.2021.614243
The Phospholipid N-Methyltransferase and Phosphatidylcholine Synthase Pathways and the ChoXWV Choline Uptake System Involved in Phosphatidylcholine Synthesis Are Widely Conserved in Most, but Not All Brucella Species
Abstract
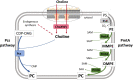
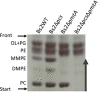
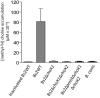
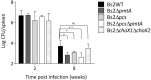
The brucellae are facultative intracellular bacteria with a cell envelope rich in phosphatidylcholine (PC). PC is abundant in eukaryotes but rare in prokaryotes, and it has been proposed that Brucella uses PC to mimic eukaryotic-like features and avoid innate immune responses in the host. Two PC synthesis pathways are known in prokaryotes: the PmtA-catalyzed trimethylation of phosphatidylethanolamine and the direct linkage of choline to CDP-diacylglycerol catalyzed by the PC synthase Pcs. Previous studies have reported that B. abortus and B. melitensis possess non-functional PmtAs and that PC is synthesized exclusively via Pcs in these strains. A putative choline transporter ChoXWV has also been linked to PC synthesis in B. abortus. Here, we report that Pcs and Pmt pathways are active in B. suis biovar 2 and that a bioinformatics analysis of Brucella genomes suggests that PmtA is only inactivated in B. abortus and B. melitensis strains. We also show that ChoXWV is active in B. suis biovar 2 and conserved in all brucellae except B. canis and B. inopinata. Unexpectedly, the experimentally verified ChoXWV dysfunction in B. canis did not abrogate PC synthesis in a PmtA-deficient mutant, which suggests the presence of an unknown mechanism for obtaining choline for the Pcs pathway in Brucella. We also found that ChoXWV dysfunction did not cause attenuation in B. suis biovar 2. The results of these studies are discussed with respect to the proposed role of PC in Brucella virulence and how differential use of the Pmt and Pcs pathways may influence the interactions of these bacteria with their mammalian hosts.
Keywords: brucella; cell envelope; choline; phosphatidylcholine; phospholipid.
Copyright © 2021 Aragón-Aranda, Palacios-Chaves, Salvador-Bescós, de Miguel, Muñoz, Vences-Guzmán, Zúñiga-Ripa, Lázaro-Antón, Sohlenkamp, Moriyón, Iriarte and Conde-Álvarez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

