Intestinal Regulatory T Cells as Specialized Tissue-Restricted Immune Cells in Intestinal Immune Homeostasis and Disease
- PMID: 34421921
- PMCID: PMC8371910
- DOI: 10.3389/fimmu.2021.716499
Intestinal Regulatory T Cells as Specialized Tissue-Restricted Immune Cells in Intestinal Immune Homeostasis and Disease
Abstract
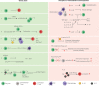
FOXP3+ regulatory T cells (Treg cells) are a specialized population of CD4+ T cells that restrict immune activation and are essential to prevent systemic autoimmunity. In the intestine, the major function of Treg cells is to regulate inflammation as shown by a wide array of mechanistic studies in mice. While Treg cells originating from the thymus can home to the intestine, the majority of Treg cells residing in the intestine are induced from FOXP3neg conventional CD4+ T cells to elicit tolerogenic responses to microbiota and food antigens. This process largely takes place in the gut draining lymph nodes via interaction with antigen-presenting cells that convert circulating naïve T cells into Treg cells. Notably, dysregulation of Treg cells leads to a number of chronic inflammatory disorders, including inflammatory bowel disease. Thus, understanding intestinal Treg cell biology in settings of inflammation and homeostasis has the potential to improve therapeutic options for patients with inflammatory bowel disease. Here, the induction, maintenance, trafficking, and function of intestinal Treg cells is reviewed in the context of intestinal inflammation and inflammatory bowel disease. In this review we propose intestinal Treg cells do not compose fixed Treg cell subsets, but rather (like T helper cells), are plastic and can adopt different programs depending on microenvironmental cues.
Keywords: IBD; homeostasis; intestinal inflammation; intestine; regulatory T (Treg) cells.
Copyright © 2021 Jacobse, Li, Rings, Samsom and Goettel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

