Efficacy of Humanized Anti-BCMA CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients With and Without Extramedullary Disease
- PMID: 34421924
- PMCID: PMC8374046
- DOI: 10.3389/fimmu.2021.720571
Efficacy of Humanized Anti-BCMA CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients With and Without Extramedullary Disease
Abstract
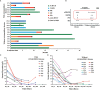
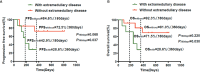
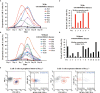
In recent years, many new treatments for relapsed/refractory (R/R) multiple myeloma (MM) have improved patient prognosis, but the prognosis of patients with extramedullary MM is still particularly poor. Therefore, more efficacious therapies and novel strategies are urgently needed for these patients. The aim of this study was to observe and compare the efficacy and safety of humanized anti-B cell maturation antigen (anti-BCMA) chimeric antigen receptor (CAR) T cell therapy in R/R MM patients with and without extramedullary disease. Seven R/R MM patients with extramedullary disease and 13 without extramedullary disease received humanized anti-BCMA CAR T cell therapy. The overall response rate was not different between patients with and without extramedullary disease. There was no difference in the progression-free survival (PFS) or overall survival (OS) rates between the two groups at 180 days, but the PFS and OS rates in patients with extramedullary disease were lower at 360 days than those in patients without extramedullary disease. Although some patients with extramedullary disease experienced further disease progression, their M protein level did not increase. We did not see this change trend of M protein in patients without extramedullary disease. However, this was not observed in patients without extramedullary disease. Among patients who responded to CAR T cell therapy, the grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxic syndrome (ICANS) were much higher among patients with extramedullary disease. In summary, R/R MM patients with extramedullary disease could benefit from humanized anti-BCMA CAR T cell therapy in the short term, although the CRS and ICANS grades were much higher in patients with extramedullary disease. Therefore, anti-BCMA CAR T cell therapy allows for a remission time for R/R MM patients with extramedullary disease, which could be maintained by bridging hematopoietic stem cell transplantation, radiotherapy, and other therapies.
Clinical trial registration: http://www.chictr.org.cn/index.aspx, identifiers ChiCTR1800017051 and ChiCTR2000033925.
Keywords: anti-B cell maturation antigen chimeric antigen receptor T; efficacy; extramedullary disease; multiple myeloma; refractory; relapsed.
Copyright © 2021 Deng, Liu, Yuan, Zhang, Cui, Li, Yuan, Wang, Wang and Deng.
Conflict of interest statement
Author JY was employed by the company Shanghai Genbase Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Figures


References
-
- Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. . Daratumumab Plus Bortezomib and Dexamethasone Versus Bortezomib and Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Analysis of CASTOR. Haematologica (2018) 103(12):2079–87. 10.3324/haematol.2018.194118 - DOI - PMC - PubMed
-
- Dimopoulos M, Weisel K, van de Donk N, Ramasamy K, Gamberi B, Streetly M, et al. . Pomalidomide Plus Low-Dose Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma and Renal Impairment: Results From a Phase II Trial. J Clin Oncol (2018) 36(20):2035–43. 10.1200/JCO.2017.76.1742 - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

