Does Shoot Apical Meristem Function as the Germline in Safeguarding Against Excess of Mutations?
- PMID: 34421954
- PMCID: PMC8374955
- DOI: 10.3389/fpls.2021.707740
Does Shoot Apical Meristem Function as the Germline in Safeguarding Against Excess of Mutations?
Abstract
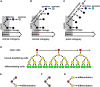
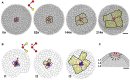
A genetic continuity of living organisms relies on the germline which is a specialized cell lineage producing gametes. Essential in the germline functioning is the protection of genetic information that is subjected to spontaneous mutations. Due to indeterminate growth, late specification of the germline, and unique longevity, plants are expected to accumulate somatic mutations during their lifetime that leads to decrease in individual and population fitness. However, protective mechanisms, similar to those in animals, exist in plant shoot apical meristem (SAM) allowing plants to reduce the accumulation and transmission of mutations. This review describes cellular- and tissue-level mechanisms related to spatio-temporal distribution of cell divisions, organization of stem cell lineages, and cell fate specification to argue that the SAM functions analogous to animal germline.
Keywords: cell division; germline; shoot apical meristem; somatic mutation; stem cell.
Copyright © 2021 Burian.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

