Visible Light-Induced Pd-Catalyzed Alkyl-Heck Reaction of Oximes
- PMID: 34422448
- PMCID: PMC8372551
- DOI: 10.1021/acscatal.1c00267
Visible Light-Induced Pd-Catalyzed Alkyl-Heck Reaction of Oximes
Abstract
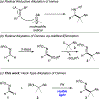
A visible light-induced palladium-catalyzed oxidative C-H alkylation of oximes has been developed. This mild protocol allows for an efficient atom economical C-C bond construction of alkyl-substituted oximes. A broad range of primary, secondary, and tertiary alkyl bromides and iodides, as well as a range of different formaldoximes, can efficiently undergo this transformation. The method features visible light-induced generation of nucleophilic hybrid alkyl Pd radical intermediates, which upon radical addition at the imine moiety and a subsequent β-hydrogen elimination deliver substituted imines.
Keywords: Heck reaction; alkylation; light-induced; oxime; palladium; radical.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Friestad GK Addition of carbon-centered radicals to imines and related compounds. Tetrahedron 2001, 57, 5461–5496.
- Miyabe H; Ueda M; Naito T Carbon-Carbon Bond Construction Based on Radical Addition to C=N Bond. Synlett 2004, 7, 1140–1157.
-
- Halland N; Anker Jørgensen K Intermolecular addition of alkyl radicals to imines in the absence and in the presence of a Lewis acid. J. Chem. Soc., Perkin Trans 12001, 1290–1295.
- Yamada K-I; Fujihara H; Yamamoto Y; Miwa Y; Taga T; Tomioka K Radical Addition of Ethers to Imines Initiated by Dimethylzinc. Org. Lett 2002, 4, 3509–3511. - PubMed
- Lee S; Kim S Enantioselective radical addition reactions to imines using binaphthol-derived chiral N-triflyl phosphor-amides. Tetrahedron Lett. 2009, 50, 3345–3348.
-
- Garrido-Castro AF; Maestro MC; Alemán J α-Functionalization of Imines via Visible Light Photoredox Catalysis. Catalysts 2020, 10, 562.
-
- Kim S; Lee IY; Yoon J-Y; Oh DH Novel Radical Reaction of Phenylsulfonyl Oxime Ethers. A Free Radical Acylation Approach. J. Am. Chem. Soc 1996, 118, 5138–5139.
- Kim S; Song H-J; Choi T-L; Yoon J-Y Tin-Free Radical Acylation Reactions with Methanesulfonyl Oxime Ether. Angew. Chem., Int. Ed 2001, 40, 2524–2526. - PubMed
- Rouquet G; Robert F; Méreau R; Castet F; Landais Y Allylsilanes in “Tin-free” Oximation, Alkenylation, and Allylation of Alkyl Halides. Chem. - Eur. J 2011, 17, 13904–13911. - PubMed
- Kamijo S; Takao G; Kamijo K; Hirota M; Tao K; Murafuji T Photo-induced Substitutive Introduction of the Aldoxime Functional Group to Carbon Chains: A Formal Formylation of Non-Acidic C(sp3)-H Bonds. Angew. Chem., Int. Ed 2016, 55, 9695–9699. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
