Tips on Raising Reliable Local Perforator Flaps
- PMID: 34422514
- PMCID: PMC8376349
- DOI: 10.1097/GOX.0000000000003673
Tips on Raising Reliable Local Perforator Flaps
Abstract
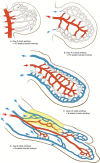
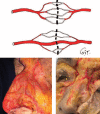
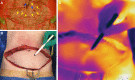
From early on in the development of plastic surgery, it was quickly realized that utilizing locally adjacent tissue, or "matching like with like," yielded superior aesthetic reconstructions to those in which the tissue was derived from a distant location. In many cases, the use of a local perforator flap is a simpler procedure with less patient morbidity and a quicker recovery from surgery. The difficulty with local perforator flaps has been locating the supplying perforators, ensuring that the flap has a robust and reliable blood supply, and that sufficient tissue is able to be transferred. The recent reappraisal of our understanding of the blood supply of the integument has allowed, for the first time, the capacity to accurately and inexpensively, without the need for "high tech equipment," locate perforators, as they emerge from the deep fascia into the overlying integument, and through a better understanding of the interconnecting anastomotic vessels between perforators reliably predict how much tissue can be safely raised on a single perforator, before surgery. Further, through the use of strategic "delay," it is possible to manipulate the interconnecting vessels between the selected perforator and its surrounding neighbors to design a flap of tissue of any dimension, composed of whatever tissue we require, and safely transfer that tissue locally, or if required, distantly, as a free flap. This article will highlight these advances, explain their relevance in raising reliable local perforator flaps, and will, where possible, call attention to any pearls and pitfalls, and how to avoid complications.
Copyright © 2021 The Author. Published by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons.
Figures

















References
-
- Gillies HD, Pilcher LS. Plastic Surgery of the Face. 1920. New York: Thieme-Stratton Corp
-
- Gillies H, Millard DR. The Principles and Art of Plastic Surgery. Boston, Toronto: Butterworth; 1957.
-
- Milton SH. Pedicled skin-flaps: the fallacy of the length: width ratio. Br J Surg. 1970;57:502–508. - PubMed
-
- Taylor GI, Pan WR. The Angiosome Concept and Tissue Transfer. New York: Thieme Medical Publishers. Vol 2:656–657.
-
- Bates D, Taylor GI, Newgreen DF. The pattern of neurovascular development in the forelimb of the quail embryo. Dev Biol. 2002;249:300–320. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
