The Ross-Konno procedure for congenital aortic stenosis
- PMID: 34422569
- PMCID: PMC8339636
- DOI: 10.21037/acs-2021-rp-31
The Ross-Konno procedure for congenital aortic stenosis
Abstract
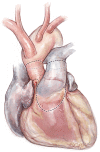
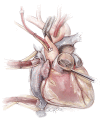
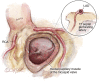
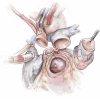
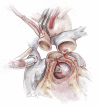
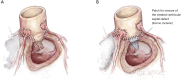
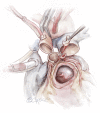
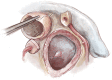
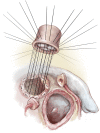
Left ventricular outflow tract (LVOT) obstruction can result from supravalvular, valvular and/or subvalvular etiologies. Congenital aortic valvular stenosis is frequently associated with aortic annular hypoplasia. Aortoventriculoplasty with pulmonary autograft, "The Ross-Konno" operation, provides more or less a radical solution to multilevel LVOT obstruction by enlarging the aortic annulus and the subvalvular area, thus relieving both valvular and subvalvular obstructions. In addition to this, the procedure carries the major advantage of having a competent autograft in the LVOT. An autograft that has the potential for growth and provides excellent quality of life without the need for anticoagulation. The procedure is most commonly performed as a complete root implantation, harvesting the coronary arteries as buttons, and harvesting the autograft with a muscle skirt to allow a single unit reconstruction of the LVOT. The procedure has been modified over time to minimize the risk of conduction tissue injury and the development of complete heart block by modifying the interventricular septal incision. The Ross-Konno procedure has changed the approach to patients with complex multilevel LVOT obstruction. However, while it can be performed early in life, one should be aware that this procedure is technically demanding and can be associated with a higher surgical risk. Nevertheless, it is considered "the ultimate solution" for those with complex multilevel LVOT obstruction.
Keywords: Ross; Ross-Konno; aortoventriculoplasty; autograft; hypoplastic aortic annulus.
2021 Annals of Cardiothoracic Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: SMS is a consultant for Cryolife, and Stryker.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
