Clinical Features and Resistance to Entecavir Monotherapy of Patients with Hepatitis B
- PMID: 34422709
- PMCID: PMC8376438
- DOI: 10.1155/2021/3259833
Clinical Features and Resistance to Entecavir Monotherapy of Patients with Hepatitis B
Abstract
Aim: Hepatitis B virus (HBV) infection is a major public health concern worldwide. Entecavir (ETV), a first-line nucleos(t)ide analogue (NA) for HBV, has a low risk of resistance. We evaluated the efficacy of ETV monotherapy, ratio of ETV-resistant, and the clinical features of patients with ETV resistance.
Methods: A total of 130 patients (72 males, 58 females; mean age, 61 ± 15 years) were divided into a NA-naïve group (n = 108) and NA-experienced group (n = 22). We examined the clinical outcomes of ETV monotherapy and associated factors. We also assessed the clinical features of 15 patients with resistance to ETV (mean, 51.0 ± 27.4 weeks).
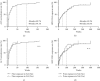
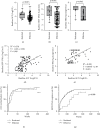
Results: Among the 130 patients, 94.1% achieved ALT normalization and 63.6% achieved serum HBV DNA negativity after ETV monotherapy for 96 weeks. Of the patients in the NA-naïve group, 93.1% and 60.4% achieved ALT normalization and HBV DNA negativity, respectively. Of the patients in the NA-experienced group, 100% and 74.9% achieved ALT normalization and HBV DNA negativity, respectively. Compared to patients on ETV continuously, 15 ETV-resistant patients had a higher baseline HBV viral load. There was a significant difference in the time to HBV DNA negativity, but not ALT normalization after ETV monotherapy in these groups. Rescue treatment with other NAs led to ALT normalization in all of these patients, but not HBV DNA negativity.
Conclusions: ETV monotherapy has a long-term clinical efficacy. While some patients especially with HBV DNA high viral load developed ETV resistance, rescue treatment led to ALT normalization in these patients.
Copyright © 2021 Hideo Takayama et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

