The Emerging Genetic Landscape of Hirschsprung Disease and Its Potential Clinical Applications
- PMID: 34422713
- PMCID: PMC8374333
- DOI: 10.3389/fped.2021.638093
The Emerging Genetic Landscape of Hirschsprung Disease and Its Potential Clinical Applications
Abstract
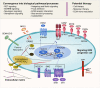
Hirschsprung disease (HSCR) is the leading cause of neonatal functional intestinal obstruction. It is a rare congenital disease with an incidence of one in 3,500-5,000 live births. HSCR is characterized by the absence of enteric ganglia in the distal colon, plausibly due to genetic defects perturbing the normal migration, proliferation, differentiation, and/or survival of the enteric neural crest cells as well as impaired interaction with the enteric progenitor cell niche. Early linkage analyses in Mendelian and syndromic forms of HSCR uncovered variants with large effects in major HSCR genes including RET, EDNRB, and their interacting partners in the same biological pathways. With the advances in genome-wide genotyping and next-generation sequencing technologies, there has been a remarkable progress in understanding of the genetic basis of HSCR in the past few years, with common and rare variants with small to moderate effects being uncovered. The discovery of new HSCR genes such as neuregulin and BACE2 as well as the deeper understanding of the roles and mechanisms of known HSCR genes provided solid evidence that many HSCR cases are in the form of complex polygenic/oligogenic disorder where rare variants act in the sensitized background of HSCR-associated common variants. This review summarizes the roadmap of genetic discoveries of HSCR from the earlier family-based linkage analyses to the recent population-based genome-wide analyses coupled with functional genomics, and how these discoveries facilitated our understanding of the genetic architecture of this complex disease and provide the foundation of clinical translation for precision and stratified medicine.
Keywords: GWAS; Hirschsprung disease; aganglionosis; common variants; genetic architecture; genetics; next-generating sequencing; rare variants.
Copyright © 2021 Karim, Tang and Tam.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

