Strong as a Hippo's Heart: Biomechanical Hippo Signaling During Zebrafish Cardiac Development
- PMID: 34422841
- PMCID: PMC8375320
- DOI: 10.3389/fcell.2021.731101
Strong as a Hippo's Heart: Biomechanical Hippo Signaling During Zebrafish Cardiac Development
Abstract
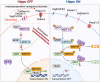
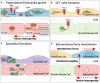
The heart is comprised of multiple tissues that contribute to its physiological functions. During development, the growth of myocardium and endocardium is coupled and morphogenetic processes within these separate tissue layers are integrated. Here, we discuss the roles of mechanosensitive Hippo signaling in growth and morphogenesis of the zebrafish heart. Hippo signaling is involved in defining numbers of cardiac progenitor cells derived from the secondary heart field, in restricting the growth of the epicardium, and in guiding trabeculation and outflow tract formation. Recent work also shows that myocardial chamber dimensions serve as a blueprint for Hippo signaling-dependent growth of the endocardium. Evidently, Hippo pathway components act at the crossroads of various signaling pathways involved in embryonic zebrafish heart development. Elucidating how biomechanical Hippo signaling guides heart morphogenesis has direct implications for our understanding of cardiac physiology and pathophysiology.
Keywords: Hippo signaling; Yap1/Wwtr1 (Taz); cardiac development; endocardium; intra-organ-communication; mechanobiology; myocardium; zebrafish.
Copyright © 2021 Bornhorst and Abdelilah-Seyfried.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

