Hyaluronic Acid Is an Effective Dermal Filler for Lip Augmentation: A Meta-Analysis
- PMID: 34422892
- PMCID: PMC8377277
- DOI: 10.3389/fsurg.2021.681028
Hyaluronic Acid Is an Effective Dermal Filler for Lip Augmentation: A Meta-Analysis
Abstract
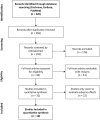
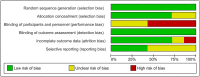
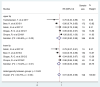
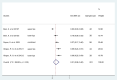
Introduction: The lips and the mouth play an indispensable role in vocalization, mastication and face aesthetics. Various noxious factors may alter and destruct the original structure, and appearance of the lips and the anatomical area surrounding the mouth. The application of hyaluronic acid (HA) may serve as a safe method for lip regeneration. Although a number of studies exist for HA effectiveness and safety, its beneficial effect is not well-established. Aim: The present meta-analysis and systematic review was performed to investigate the effectiveness of HA on lip augmentation. We also investigated the types and nature of adverse effects (AEs) of HA application. Methods: We reported our meta-analysis in accordance with the PRISMA Statement. PROSPERO protocol registration: CRD42018102899. We performed the systematic literature search in CENTRAL, Embase, and MEDLINE. Randomized controlled trials, cohort studies, case series and case reports were included. The untransformed proportion (random-effects, DerSimonian-Laird method) of responder rate to HA injection was calculated. For treatment related AEs descriptive statistics were used. Results: The systematic literature search yielded 32 eligible records for descriptive statistics and 10 records for quantitative synthesis. The results indicated that the overall estimate of responders (percentage of subjects with increased lip fullness by one point or higher) was 91% (ES = 0.91, 95% CI:0.85-0.96) 2 months after injection. The rate of responders was 74% (ES = 0.74, 95% CI:0.66-0.82) and 46% (ES = 0.46, 95% CI:0.28-0.65) after 6 and 12 months, respectively. We included 1,496 participants for estimating the event rates of AEs. The most frequent treatment-related AEs were tenderness (88.8%), injection site swelling (74.3%) and bruising (39.5%). Rare AEs included foreign body granulomas (0.6%), herpes labialis (0.6%) and angioedema (0.3%). Conclusion: Our meta-analysis revealed that lip augmentation with injectable HA is an efficient method for increasing lip fullness for at least up to 6 months after augmentation. Moreover, we found that most AEs of HA treatment were mild or moderate, but a small number of serious adverse effects were also found. In conclusion, further well-designed RCTs are still needed to make the presently available evidence stronger.
Keywords: adverse effects; dermal filler; effectiveness; hyaluronic acid; lip augmentation.
Copyright © 2021 Czumbel, Farkasdi, Gede, Mikó, Csupor, Lukács, Gaál, Kiss, Hegyi and Varga.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Effectiveness and safety of hyaluronic acid fillers used to enhance overall lip fullness: A systematic review of clinical studies.J Cosmet Dermatol. 2019 Apr;18(2):436-443. doi: 10.1111/jocd.12861. Epub 2019 Jan 12. J Cosmet Dermatol. 2019. PMID: 30636365
-
Assessment of the Efficacy and Safety of Hyaluronic Acid Gel Injection in the Restoration of Fullness of the Upper Lips.J Cutan Aesthet Surg. 2017 Apr-Jun;10(2):101-105. doi: 10.4103/JCAS.JCAS_115_16. J Cutan Aesthet Surg. 2017. PMID: 28852297 Free PMC article.
-
Delayed Granulomas as a Complication Secondary to Lip Augmentation with Dermal Fillers: A Systematic Review.Surg J (N Y). 2022 Mar 3;8(1):e69-e79. doi: 10.1055/s-0042-1743524. eCollection 2022 Jan. Surg J (N Y). 2022. PMID: 35252562 Free PMC article.
-
A Multi-Center, Open-Label, Prospective Study of Cannula Injection of Small-Particle Hyaluronic Acid Plus Lidocaine (SPHAL) for Lip Augmentation.J Drugs Dermatol. 2018 Jan 1;17(1):10-16. J Drugs Dermatol. 2018. PMID: 29320583
-
Adverse Events Reported From Hyaluronic Acid Dermal Filler Injections to the Facial Region: A Systematic Review and Meta-Analysis.Cureus. 2023 Apr 29;15(4):e38286. doi: 10.7759/cureus.38286. eCollection 2023 Apr. Cureus. 2023. PMID: 37261136 Free PMC article. Review.
Cited by
-
Clinical Efficacy and Safety of a Highly Purified Polynucleotide for Dry and Chapped Lips: A Prospective, Multicenter Study.J Cosmet Dermatol. 2025 May;24(5):e70224. doi: 10.1111/jocd.70224. J Cosmet Dermatol. 2025. PMID: 40402866 Free PMC article.
-
Development and Characterization of PEGylated Poly D,L-Lactic Acid Nanoparticles for Skin Rejuvenation.Nanomaterials (Basel). 2025 Mar 20;15(6):470. doi: 10.3390/nano15060470. Nanomaterials (Basel). 2025. PMID: 40137643 Free PMC article.
-
Long-term Efficacy and Safety of a Hyaluronic Acid-Based Dermal Filler With Tri-Hyal Technology to Enhance Lip Volume.Aesthet Surg J Open Forum. 2025 Jan 28;7:ojae110. doi: 10.1093/asjof/ojae110. eCollection 2025. Aesthet Surg J Open Forum. 2025. PMID: 40309237 Free PMC article.
-
A prospective multicenter clinical trial evaluating the efficacy and safety of a hyaluronic acid-based filler with Tri-Hyal technology in the treatment of lips and the perioral area.J Cosmet Dermatol. 2023 Feb;22(2):464-472. doi: 10.1111/jocd.15169. Epub 2022 Jul 31. J Cosmet Dermatol. 2023. PMID: 35718985 Free PMC article.
-
Botulinum Toxin Type A and Hyaluronic Acid Dermal Fillers in Dentistry: A Systematic Review of Clinical Application and Indications.J Clin Med Res. 2024 Jun;16(6):273-283. doi: 10.14740/jocmr5202. Epub 2024 Jun 30. J Clin Med Res. 2024. PMID: 39027812 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

