Inhibition of Rac1 GTPase Decreases Vascular Oxidative Stress, Improves Endothelial Function, and Attenuates Atherosclerosis Development in Mice
- PMID: 34422919
- PMCID: PMC8377253
- DOI: 10.3389/fcvm.2021.680775
Inhibition of Rac1 GTPase Decreases Vascular Oxidative Stress, Improves Endothelial Function, and Attenuates Atherosclerosis Development in Mice
Abstract
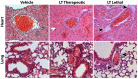
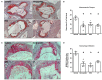
Aims: Oxidative stress and inflammation contribute to atherogenesis. Rac1 GTPase regulates pro-oxidant NADPH oxidase activity, reactive oxygen species (ROS) formation, actin cytoskeleton organization and monocyte adhesion. We investigated the vascular effects of pharmacological inhibition of Rac1 GTPase in mice. Methods and Results: We treated wild-type and apolipoprotein E-deficient (ApoE-/-) mice with Clostridium sordellii lethal toxin (LT), a Rac1 inhibitor, and assessed vascular oxidative stress, expression and activity of involved proteins, endothelial function, macrophage infiltration, and atherosclerosis development. LT-treated wild-type mice displayed decreased vascular NADPH oxidase activity and ROS production. Therapeutic LT doses had no impact on behavior, food intake, body weight, heart rate, blood pressure, vascular and myocardial function, differential blood count, and vascular permeability. ApoE-/- mice were fed a cholesterol-rich diet and were treated with LT or vehicle. LT treatment led to decreased aortic Rac1 GTPase activity, NADPH oxidase activity and ROS production, but had no impact on expression and membrane translocation of NADPH oxidase subunits and RhoA GTPase activity. LT-treated mice showed improved aortic endothelium-dependent vasodilation, attenuated atherosclerotic lesion formation and reduced macrophage infiltration of atherosclerotic plaques. Concomitant treatment of cholesterol-fed ApoE-/- mice with LT, the specific synthetic Rac1 inhibitor NSC 23766 or simvastatin comparably reduced aortic Rac1 activity, NADPH oxidase activity, oxidative stress, endothelial dysfunction, atherosclerosis development, and macrophage infiltration. Conclusions: These findings identify an important role of the small GTPase Rac1 in atherogenesis and provide a potential target for anti-atherosclerotic therapy.
Keywords: GTPases; Rac1; atherosclerosis; endothelial function; free radicals; oxidative stress.
Copyright © 2021 Zimmer, Goody, Oelze, Ghanem, Mueller, Laufs, Daiber, Jansen, Nickenig and Wassmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

