Materials with Hierarchical Porosity Enhance the Stability of Infused Ionic Liquid Films
- PMID: 34423203
- PMCID: PMC8374917
- DOI: 10.1021/acsomega.1c02405
Materials with Hierarchical Porosity Enhance the Stability of Infused Ionic Liquid Films
Abstract
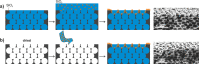
Defined surface functionalities can control the properties of a material. The layer-by-layer method is an experimentally simple yet very versatile method to coat a surface with nanoscale precision. The method is widely used to either control the chemical properties of the surface via the introduction of functional moieties bound to the polymer or create nanoscale surface topographies if one polymeric species is replaced by a colloidal dispersion. Such roughness can enhance the stability of a liquid film on top of the surface by capillary adhesion. Here, we investigate whether a similar effect allows an increased retention of liquid films within a porous surface and thus potentially increases the stability of ionic liquid films infused within a porous matrix in the supported ionic liquid-phase catalysis. The complex geometry of the porous material, long diffusion pathways, and small sizes of necks connecting individual pores all contribute to difficulties to reliably coat the required porous materials. We optimize the coating process to ensure uniform surface functionalization via two steps. Diffusion limitations are overcome by force-wetting the pores, which transports the functional species convectively into the materials. Electrostatic repulsion, which can limit pore accessibility, is mitigated by the addition of electrolytes to screen charges. We introduce nanoscale topography in microscale porous SiC monoliths to enhance the retention of an ionic liquid film. We use γ-Al2O3 to coat monoliths and test the retention of 1-butyl-2,3-dimethylimidazolium chloride under exposure to a continuous gas stream, a setup commonly used in the water-gas shift reaction. Our study showcases that a hierarchical topography can improve the stability of impregnated ionic liquid films, with a potential advantage of improved supported ionic liquid-phase catalysis.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Decher G.; Hong J. D. Buildup Of Ultrathin Multilayer Films By A Self-Assembly Process .1. Consecutive Adsorption Of Anionic And Cationic Bipolar Amphiphiles On Charged Surfaces. Makromol. Chemie-Macromolecular Symp. 1991, 46, 321–327. 10.1002/Masy.19910460145. - DOI
-
- Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. 10.1126/Science.277.5330.1232. - DOI
-
- Fou A. C.; Rubner M. F. Molecular-Level Processing Of Conjugated Polymers. 2. Layer-By-Layer Manipulation Of In-Situ Polymerized P-Type Doped Conducting Polymers. Macromolecules 1995, 28, 7115–7120. 10.1021/Ma00125a013. - DOI
LinkOut - more resources
Full Text Sources

