Cardiac Magnetic Resonance Reveals Incipient Cardiomyopathy Traits in Adult Patients With Phenylketonuria
- PMID: 34423658
- PMCID: PMC8649272
- DOI: 10.1161/JAHA.120.020351
Cardiac Magnetic Resonance Reveals Incipient Cardiomyopathy Traits in Adult Patients With Phenylketonuria
Abstract
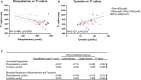
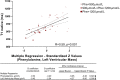
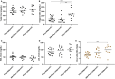
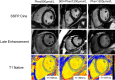
Background Phenylketonuria is the most common inborn error of amino acid metabolism, where oxidative stress and collateral metabolic abnormalities are likely to cause cardiac structural and functional modifications. We aim herein to characterize the cardiac phenotype of adult subjects with phenylketonuria using advanced cardiac imaging. Methods and Results Thirty-nine adult patients with phenylketonuria (age, 30.5±8.7 years; 10-year mean phenylalanine concentration, 924±330 µmol/L) and 39 age- and sex-matched healthy controls were investigated. Participants underwent a comprehensive cardiac magnetic resonance and echocardiography examination. Ten-year mean plasma levels of phenylalanine and tyrosine were used to quantify disease activity and adherence to treatment. Patients with phenylketonuria had thinner left ventricular walls (septal end-diastolic thickness, 7.0±17 versus 8.8±1.7 mm [P<0.001]; lateral thickness, 6.1±1.4 versus 6.8±1.2 mm [P=0.004]), more dilated left ventricular cavity (end-diastolic volume, 87±14 versus 80±14 mL/m2 [P=0.0178]; end-systolic volume, 36±9 versus 29±8 mL/m2 [P<0.001]), lower ejection fraction (59±6% versus 64±6% [P<0.001]), reduced systolic deformation (global circumferential strain, -29.9±4.2 % versus -32.2±5.0 % [P=0.027]), and lower left ventricular mass (38.2±7.9 versus 47.8±11.0 g/m2 [P<0.001]). T1 native values were decreased (936±53 versus 996±26 ms [P<0.001]), with particular low values in patients with phenylalanine >1200 µmol/L (909±48 ms). Both mean phenylalanine (P=0.013) and tyrosine (P=0.035) levels were independently correlated with T1; and in a multiple regression model, higher phenylalanine levels and higher left ventricular mass associate with lower T1. Conclusions Cardiac phenotype of adult patients with phenylketonuria reveals some traits of an early-stage cardiomyopathy. Regular cardiology follow-up, tighter therapeutic control, and prophylaxis of cardiovascular risk factors, in particular dyslipidemia, are recommended.
Keywords: T1 native; cardiac magnetic resonance; cardiomyopathy; dyslipidemia; phenylketonuria.
Conflict of interest statement
Dr Pieske reports having received consultancy and lecture honoraria from Bayer Daiichi Sankyo, MSD, Novartis, Sanofi‐Aventis, Stealth Peptides, and Vifor Pharma; and editor honoraria from the
Figures

References
-
- Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

