Cell therapy for cartilage repair
- PMID: 34423830
- PMCID: PMC8589441
- DOI: 10.1042/ETLS20210015
Cell therapy for cartilage repair
Abstract
Regenerative medicine, using cells as therapeutic agents for the repair or regeneration of tissues and organs, offers great hope for the future of medicine. Cell therapy for treating defects in articular cartilage has been an exemplar of translating this technology to the clinic, but it is not without its challenges. These include applying regulations, which were designed for pharmaceutical agents, to living cells. In addition, using autologous cells as the therapeutic agent brings additional costs and logistical challenges compared with using allogeneic cells. The main cell types used in treating chondral or osteochondral defects in joints to date are chondrocytes and mesenchymal stromal cells derived from various sources such as bone marrow, adipose tissue or umbilical cord. This review discusses some of their biology and pre-clinical studies before describing the most pertinent clinical trials in this area.
Keywords: cartilage; cell therapy; chondrocytes; mesenchymal stem cell; osteoarthritis; regenerative medicine.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
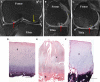


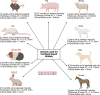
References
-
- Hunter, W.V.I. (1743) Of the structure and diseases of articulating cartilages. Philos. Trans. R. Soc. Lond. 42, 514–521 10.1098/rstl.1742.0079 - DOI
-
- Registry, N.J. (2020) 17th Annual Report 2020 https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2017th%20Ann...
-
- OARSI White Paper- OA as a Serious Disease | Osteoarthritis research society international (oarsi)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
