Supported CuII Single-Ion Catalyst for Total Carbon Utilization of C2 and C3 Biomass-Based Platform Molecules in the N-Formylation of Amines
- PMID: 34423878
- PMCID: PMC9292173
- DOI: 10.1002/chem.202102300
Supported CuII Single-Ion Catalyst for Total Carbon Utilization of C2 and C3 Biomass-Based Platform Molecules in the N-Formylation of Amines
Abstract
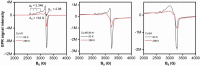
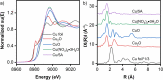
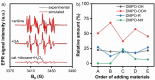
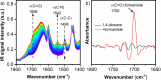
The shift from fossil carbon sources to renewable ones is vital for developing sustainable chemical processes to produce valuable chemicals. In this work, value-added formamides were synthesized in good yields by the reaction of amines with C2 and C3 biomass-based platform molecules such as glycolic acid, 1,3-dihydroxyacetone and glyceraldehyde. These feedstocks were selectively converted by catalysts based on Cu-containing zeolite 5A through the in situ formation of carbonyl-containing intermediates. To the best of our knowledge, this is the first example in which all the carbon atoms in biomass-based feedstocks could be amidated to produce formamide. Combined catalyst characterization results revealed preferably single CuII sites on the surface of Cu/5A, some of which form small clusters, but without direct linking via oxygen bridges. By combining the results of electron paramagnetic resonance (EPR) spin-trapping, operando attenuated total reflection (ATR) IR spectroscopy and control experiments, it was found that the formation of formamides might involve a HCOOH-like intermediate and . NHPh radicals, in which the selective formation of . OOH radicals might play a key role.
Keywords: C−C bond cleavage; amines; biomass-based feedstocks; electron paramagnetic resonance spectroscopy; formylation; selective oxidation.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Cho E. J., Trinh L. T. P., Song Y., Lee Y. G., Bae H.-J., Bioresour. Technol. 2020, 298, 122386; - PubMed
-
- Huber G. W., Iborra S., Corma A., Chem. Rev. 2006, 106, 4044–4098; - PubMed
-
- Chheda J. N., Huber G. W., Dumesic J. A., Angew. Chem. Int. Ed. 2007, 46, 7164–7183; - PubMed
- Angew. Chem. 2007, 119, 7298–7318.
-
- Göransson K., Söderlind U., He J., Zhang W., Renewable Sustainable Energy Rev. 2011, 15, 482–492.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

