Genomic insights into the diversity, virulence and resistance of Klebsiella pneumoniae extensively drug resistant clinical isolates
- PMID: 34424159
- PMCID: PMC8549359
- DOI: 10.1099/mgen.0.000613
Genomic insights into the diversity, virulence and resistance of Klebsiella pneumoniae extensively drug resistant clinical isolates
Abstract
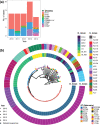
Klebsiella pneumoniae has been implicated in wide-ranging nosocomial outbreaks, causing severe infections without effective treatments due to antibiotic resistance. Here, we performed genome sequencing of 70 extensively drug resistant clinical isolates, collected from Brasília's hospitals (Brazil) between 2010 and 2014. The majority of strains (60 out of 70) belonged to a single clonal complex (CC), CC258, which has become distributed worldwide in the last two decades. Of these CC258 strains, 44 strains were classified as sequence type 11 (ST11) and fell into two distinct clades, but no ST258 strains were found. These 70 strains had a pan-genome size of 10 366 genes, with a core-genome size of ~4476 genes found in 95 % of isolates. Analysis of sequences revealed diverse mechanisms of resistance, including production of multidrug efflux pumps, enzymes with the same target function but with reduced or no affinity to the drug, and proteins that protected the drug target or inactivated the drug. β-Lactamase production provided the most notable mechanism associated with K. pneumoniae. Each strain presented two or three different β-lactamase enzymes, including class A (SHV, CTX-M and KPC), class B and class C AmpC enzymes, although no class D β-lactamase was identified. Strains carrying the NDM enzyme involved three different ST types, suggesting that there was no common genetic origin.
Keywords: CC258; Klebsiella pneumoniae; ST11; XDR; pan-genome; β-lactamase.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
-
- Azevedo PAA, Furlan JPR, Goncalves GB, Gomes CN, Goulart RDS, et al. Molecular characterisation of multidrug-resistant Klebsiella pneumoniae belonging to CC258 isolated from outpatients with urinary tract infection in Brazil. J Glob Antimicrob Resist. 2019;18:74–79. doi: 10.1016/j.jgar.2019.01.025. - DOI - PubMed
-
- Martin WJ, Yu PK, Washington JA. Epidemiologic significance of Klebsiella pneumoniae: a 3-month study. Mayo Clin Proc. 1971;46:785–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

