Paclitaxel and mortality in patients with claudication and de novo femoropopliteal lesions: a historical cohort study
- PMID: 34424424
- PMCID: PMC8382808
- DOI: 10.1186/s42155-021-00255-1
Paclitaxel and mortality in patients with claudication and de novo femoropopliteal lesions: a historical cohort study
Abstract
Objective: To compare the mortality rates of patients with claudication and de novo femoropopliteal lesions treated with and without paclitaxel coated devices (PCD).
Background: A recent meta-analysis, mostly including patients with claudication and de novo femoropopliteal lesions but also with recurrent stenoses and critical limb ischemia, has shown a significant excess mortality in patients treated with PCD.
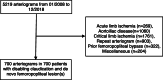
Methods: Comparison of two historical cohorts of patients presenting with claudication and de novo femoropopliteal lesions treated with and without PCD between 2008 and 2018.
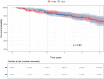
Results: After review of 5219 arteriograms in patients presenting with peripheral artery disease, 700 consecutive patients were included consisting in 72.6% of male (n = 508). Mean age was 68.1 ± 8.5 years. 45.7% of the patients (n = 320) had a treatment including a PCD. Mean femoropopliteal lesion length was 123 ± 91 mm including 44.6% of occlusions. Patients of the control group were censored at crossover to paclitaxel when applicable. Mortality rates at 1, 2 and 5 years were 4.6%, 7.5%, 19.4% and 1.6%, 6.2%, 16.6% in the non-PCD and PCD groups respectively. The relative risks of death when using PCD were 0.35 (p = 0.03), 0.83 (p = NS) and 0.86 (p = NS) at 1, 2 and 5 years respectively.
Conclusion: There was no excess mortality in patients with claudication and de novo femoropopliteal lesions treated with paclitaxel coated devices at 1, 2 and 5 years of follow-up in this cohort. The current study suggests that additional prospective randomized studies properly powered to study mortality are necessary.
Keywords: Claudication; Drug coated balloon; Drug eluting stent; Mortality; Paclitaxel; Peripheral artery disease.
© 2021. The Author(s).
Conflict of interest statement
Gerald Gahide is medical consultant for Boston Scientific. The other authors declare that they have no competing interests.
Figures

References
-
- Böhme T, Noory E, Beschorner U, Jacques B, Bürgelin K, Macharzina R, Gebauer E, Cheung F, Lechner P, Nührenberg T, Zeller T. Evaluation of mortality following paclitaxel drug-coated balloon angioplasty of Femoropopliteal lesions in the real world. JACC Cardiovasc Interv. 2020;13(17):2052–2061. doi: 10.1016/j.jcin.2020.04.050. - DOI - PubMed
-
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the Femoropopliteal artery of the leg: a systematic review and Meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7(24):e011245. doi: 10.1161/JAHA.118.011245. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
