BEX2 is required for maintaining dormant cancer stem cell in hepatocellular carcinoma
- PMID: 34424582
- PMCID: PMC8586677
- DOI: 10.1111/cas.15115
BEX2 is required for maintaining dormant cancer stem cell in hepatocellular carcinoma
Abstract
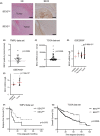
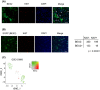
Cancer stem cells (CSCs) are responsible for therapy resistance and share several properties with normal stem cells. Here, we show that brain-expressed X-linked gene 2 (BEX2), which is essential for dormant CSCs in cholangiocarcinoma, is highly expressed in human hepatocellular carcinoma (HCC) lesions compared with the adjacent normal lesions and that in 41 HCC cases the BEX2high expression group is correlated with a poor prognosis. BEX2 localizes to Ki67-negative (nonproliferative) cancer cells in HCC tissues and is highly expressed in the dormant fraction of HCC cell lines. Knockdown of BEX2 attenuates CSC phenotypes, including sphere formation ability and aldefluor activity, and BEX2 overexpression enhances these phenotypes. Moreover, BEX2 knockdown increases cisplatin sensitivity, and BEX2 expression is induced by cisplatin treatment. Taken together, these data suggest that BEX2 induces dormant CSC properties and affects the prognosis of patients with HCC.
Keywords: BEX2; cancer stem cell; dormant; hepatocellular carcinoma; prognosis.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
There are no financial conflicts of interest.
Figures



References
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301‐1314. - PubMed
-
- Global Cancer Observatory [Internet]. [cited 2020 Aug 17]. Available from: https://gco.iarc.fr/
-
- Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153‐166. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

