Pig-to-baboon lung xenotransplantation: Extended survival with targeted genetic modifications and pharmacologic treatments
- PMID: 34424601
- PMCID: PMC10292947
- DOI: 10.1111/ajt.16809
Pig-to-baboon lung xenotransplantation: Extended survival with targeted genetic modifications and pharmacologic treatments
Abstract
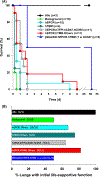
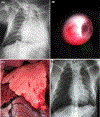
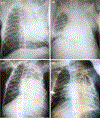
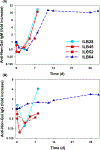
Galactosyl transferase knock-out pig lungs fail rapidly in baboons. Based on previously identified lung xenograft injury mechanisms, additional expression of human complement and coagulation pathway regulatory proteins, anti-inflammatory enzymes and self-recognition receptors, and knock-down of the β4Gal xenoantigen were tested in various combinations. Transient life-supporting GalTKO.hCD46 lung function was consistently observed in association with either hEPCR (n = 15), hTBM (n = 4), or hEPCR.hTFPI (n = 11), but the loss of vascular barrier function in the xenograft and systemic inflammation in the recipient typically occurred within 24 h. Co-expression of hEPCR and hTBM (n = 11) and additionally blocking multiple pro-inflammatory innate and adaptive immune mechanisms was more consistently associated with survival >1 day, with one recipient surviving for 31 days. Combining targeted genetic modifications to the lung xenograft with selective innate and adaptive immune suppression enables prolonged initial life-supporting lung function and extends lung xenograft recipient survival, and illustrates residual barriers and candidate treatment strategies that may enable the clinical application of other organ xenografts.
Keywords: animal models: nonhuman primate; basic (laboratory) research/science; genetics; graft survival; immunobiology; lung transplantation/pulmonology; translational research/science; xenoantibody; xenotransplantation.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures


References
-
- Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018;564(7736):430–433. - PubMed
-
- Nguyen BN, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg 2007;133(5):1354–1363. - PubMed
-
- Burdorf L, Azimzadeh AM, Pierson RN 3rd. Progress and challenges in lung xenotransplantation: an update. Curr Opin Organ Transplant 2018;23(6):621–627. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

