The tardigrade Hypsibius exemplaris has the active mitochondrial alternative oxidase that could be studied at animal organismal level
- PMID: 34424897
- PMCID: PMC8382173
- DOI: 10.1371/journal.pone.0244260
The tardigrade Hypsibius exemplaris has the active mitochondrial alternative oxidase that could be studied at animal organismal level
Abstract
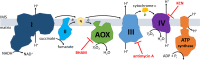
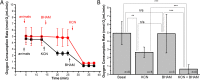
Mitochondrial alternative oxidase (AOX) is predicted to be present in mitochondria of several invertebrate taxa including tardigrades. Independently of the reason concerning the enzyme occurrence in animal mitochondria, expression of AOX in human mitochondria is regarded as a potential therapeutic strategy. Till now, relevant data were obtained due to heterologous AOX expression in cells and animals without natively expressed AOX. Application of animals natively expressing AOX could importantly contribute to the research. Thus, we decided to investigate AOX activity in intact specimens of the tardigrade Hypsibius exemplaris. We observed that H. exemplaris specimens' tolerance to the blockage of the mitochondrial respiratory chain (MRC) cytochrome pathway was diminished in the presence of AOX inhibitor and the inhibitor-sensitive respiration enabled the tardigrade respiration under condition of the blockage. Importantly, these observations correlated with relevant changes of the mitochondrial inner membrane potential (Δψ) detected in intact animals. Moreover, detection of AOX at protein level required the MRC cytochrome pathway blockage. Overall, we demonstrated that AOX activity in tardigrades can be monitored by the animals' behavior observation as well as by measurement of intact specimens' whole-body respiration and Δψ. Furthermore, it is also possible to check the impact of the MRC cytochrome pathway blockage on AOX level as well as AOX inhibition in the absence of the blockage on animal functioning. Thus, H. exemplaris could be consider as a whole-animal model suitable to study AOX.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Mitochondria-Derived Reactive Oxygen Species Regulation of Tardigrade Osmobiosis Revealed by Proteomics of Hypsibius exemplaris.J Proteome Res. 2025 Aug 1;24(8):4098-4113. doi: 10.1021/acs.jproteome.5c00222. Epub 2025 Jul 7. J Proteome Res. 2025. PMID: 40623961
-
Biogenesis of mitochondria in cauliflower (Brassica oleracea var. botrytis) curds subjected to temperature stress and recovery involves regulation of the complexome, respiratory chain activity, organellar translation and ultrastructure.Biochim Biophys Acta. 2015 Apr-May;1847(4-5):399-417. doi: 10.1016/j.bbabio.2015.01.005. Epub 2015 Jan 21. Biochim Biophys Acta. 2015. PMID: 25617518
-
Evidence of an alternative oxidase pathway for mitochondrial respiration in the scuticociliate Philasterides dicentrarchi.Protist. 2013 Nov;164(6):824-36. doi: 10.1016/j.protis.2013.09.003. Epub 2013 Oct 11. Protist. 2013. PMID: 24211656
-
Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants.Int J Mol Sci. 2013 Mar 26;14(4):6805-47. doi: 10.3390/ijms14046805. Int J Mol Sci. 2013. PMID: 23531539 Free PMC article. Review.
-
[Alternative oxidase - never ending story].Postepy Biochem. 2016;62(2):138-148. Postepy Biochem. 2016. PMID: 28132465 Review. Polish.
Cited by
-
The effect of hypomagnetic field on survival and mitochondrial functionality of active Paramacrobiotus experimentalis females and males of different age.Front Physiol. 2023 Sep 8;14:1253483. doi: 10.3389/fphys.2023.1253483. eCollection 2023. Front Physiol. 2023. PMID: 37745239 Free PMC article.
-
The effects of potassium cyanide on tardigrade Paramacrobiotus experimentalis.Sci Rep. 2025 Jul 20;15(1):26334. doi: 10.1038/s41598-025-05927-9. Sci Rep. 2025. PMID: 40685386 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

