Organized primary human papillomavirus-based cervical screening: A randomized healthcare policy trial
- PMID: 34424907
- PMCID: PMC8423359
- DOI: 10.1371/journal.pmed.1003748
Organized primary human papillomavirus-based cervical screening: A randomized healthcare policy trial
Abstract
Background: Clinical trials in the research setting have demonstrated that primary human papillomavirus (HPV)-based screening results in greater protection against cervical cancer compared with cytology, but evidence from real-life implementation was missing. To evaluate the effectiveness of HPV-based cervical screening within a real-life screening program, the organized, population-based cervical screening program in the capital region of Sweden offered either HPV- or cytology-based screening in a randomized manner through a randomized healthcare policy (RHP).
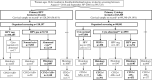
Methods and findings: A total of 395,725 women aged 30 to 64 years that were invited for their routine cervical screening visit were randomized without blinding to either cytology-based screening with HPV triage (n = 183,309) or HPV-based screening, with cytology triage (n = 212,416 women) between September 1, 2014 and September 30, 2016 and follow-up through June 30, 2017. The main outcome was non-inferior detection rate of cervical intraepithelial neoplasia grade 2 or worse (CIN2+). Secondary outcomes included superiority in CIN2+ detection, screening attendance, and referral to histology. In total, 120,240 had a cervical screening sample on record in the study period in the HPV arm and 99,340 in the cytology arm and were followed for the outcomes of interest. In per-protocol (PP) analyses, the detection rate of CIN2+ was 1.03% (95% confidence interval (CI) 0.98 to 1.10) in the HPV arm and 0.93% (0.87 to 0.99) in the cytology arm (p for non-inferiority <0.0001; odds ratio (OR) 1.11 (95% CI 1.02 to 1.22)). There were 46 cervical cancers detected in the HPV arm (0.04% (0.03 to 0.06)) and 48 cancers detected in the cytology arm (0.05% (0.04 to 0.07)) (p for non-inferiority <0.0001; OR 0.79 (0.53 to 1.18)). Intention-to-screen (ITS) analyses found few differences. In the HPV arm, there was a modestly increased attendance after new invitations (68.56% (68.31 to 68.80) vs. 67.71% (67.43 to 67.98); OR 1.02 (1.00 to 1.03)) and increased rate of referral with completed biopsy (3.89% (3.79 to 4.00) vs. 3.53% (3.42 to 3.65); OR 1.10 (1.05 to 1.15)). The main limitations of this analysis are that only the baseline results are presented, and there was an imbalance in invitations between the study arms.
Conclusions: In this study, we observed that a real-life RHP of primary HPV-based screening was acceptable and effective when evaluated against cytology-based screening, as indicated by comparable participation, referral, and detection rates.
Trial registration: ClinicalTrials.gov NCT01511328.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Evaluation of primary HPV-based cervical screening among older women: Long-term follow-up of a randomized healthcare policy trial in Sweden.PLoS Med. 2024 Dec 19;21(12):e1004505. doi: 10.1371/journal.pmed.1004505. eCollection 2024 Dec. PLoS Med. 2024. PMID: 39700313 Free PMC article. Clinical Trial.
-
Randomised healthcare policy evaluation of organised primary human papillomavirus screening of women aged 56-60.BMJ Open. 2017 May 30;7(5):e014788. doi: 10.1136/bmjopen-2016-014788. BMJ Open. 2017. PMID: 28566363 Free PMC article. Clinical Trial.
-
Human papillomavirus-based cervical screening and long-term cervical cancer risk: a randomised health-care policy trial in Sweden.Lancet Public Health. 2024 Nov;9(11):e886-e895. doi: 10.1016/S2468-2667(24)00218-4. Lancet Public Health. 2024. PMID: 39486904 Clinical Trial.
-
Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries.Vaccine. 2008 Aug 19;26 Suppl 10:K29-41. doi: 10.1016/j.vaccine.2008.06.019. Vaccine. 2008. PMID: 18847555 Review.
-
Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans.Clin Microbiol Infect. 2020 May;26(5):579-583. doi: 10.1016/j.cmi.2019.09.006. Epub 2019 Sep 17. Clin Microbiol Infect. 2020. PMID: 31539637 Review.
Cited by
-
Atypical glandular cells and development of cervical cancer: Population-based cohort study.Int J Cancer. 2022 Dec 1;151(11):2012-2019. doi: 10.1002/ijc.34242. Epub 2022 Aug 27. Int J Cancer. 2022. PMID: 36029205 Free PMC article.
-
Divergent effects of switching from cytology to HPV-based screening in the Nordic countries.Eur J Public Health. 2024 Apr 3;34(2):354-360. doi: 10.1093/eurpub/ckad225. Eur J Public Health. 2024. PMID: 38261374 Free PMC article.
-
Incidence of oncogenic HPV infection in women with and without mental illness: A population-based cohort study in Sweden.PLoS Med. 2024 Mar 25;21(3):e1004372. doi: 10.1371/journal.pmed.1004372. eCollection 2024 Mar. PLoS Med. 2024. PMID: 38527071 Free PMC article.
-
Impact of cervical screening by human papillomavirus genotype: Population-based estimations.PLoS Med. 2023 Oct 27;20(10):e1004304. doi: 10.1371/journal.pmed.1004304. eCollection 2023 Oct. PLoS Med. 2023. PMID: 37889928 Free PMC article.
-
Population-Based Age-Period-Cohort Analysis of Declining Human Papillomavirus Prevalence.J Infect Dis. 2025 Apr 15;231(4):e638-e649. doi: 10.1093/infdis/jiaf032. J Infect Dis. 2025. PMID: 39841153 Free PMC article.
References
-
- World Health Organization. Comprehensive cervical cancer control: a guide to essential practice. 2nd ed. Geneva, Switzerland: World Health Organization; 2014. - PubMed
-
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, vol 100b, Human Papillomaviruses. Lyon, France: International Agency for Research on Cancer; 2012.
-
- von Karsa L, Arbyn M, De Vuyst H, Dillner J, Dillner L, Franceschi S, et al.. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015;1:22–31.
-
- Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, et al.. European Guidelines for Quality Assurance in Cervical Cancer Screening. 2nd ed. Luxembourg: Office for Official Publications of the European Communities; 2008.

