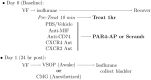
Intravesical CD74 and CXCR4, macrophage migration inhibitory factor (MIF) receptors, mediate bladder pain
- PMID: 34424927
- PMCID: PMC8382170
- DOI: 10.1371/journal.pone.0255975
Intravesical CD74 and CXCR4, macrophage migration inhibitory factor (MIF) receptors, mediate bladder pain
Abstract
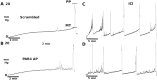
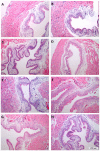
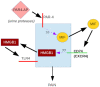
Background: Activation of intravesical protease activated receptor 4 (PAR4) leads to release of urothelial macrophage migration inhibitory factor (MIF). MIF then binds to urothelial MIF receptors to release urothelial high mobility group box-1 (HMGB1) and elicit bladder hyperalgesia. Since MIF binds to multiple receptors, we investigated the contribution of individual urothelial MIF receptors to PAR4-induced HMGB1 release in vivo and in vitro and bladder pain in vivo.
Methodology/principal findings: We tested the effect of intravesical pre-treatment with individual MIF or MIF receptor (CD74, CXCR4, CXCR2) antagonists on PAR4-induced HMGB1 release in vivo (female C57/BL6 mice) and in vitro (primary human urothelial cells) and on PAR4-induced bladder hyperalgesia in vivo (mice). In mice, PAR4 induced HMGB1 release and bladder hyperalgesia through activation of intravesical MIF receptors, CD74 and CXCR4. CXCR2 was not involved in these effects. In primary urothelial cells, PAR4-induced HMGB1 release through activation of CD74 receptors. Micturition parameters in mice were not changed by any of the treatments.
Conclusions/significance: Urothelial MIF receptors CD74 and CXCR4 mediate bladder pain through release of urothelial HMGB1. This mechanism may set up persistent pain loops in the bladder and warrants further investigation. Urothelial CD74 and CXCR4 may provide novel targets for interrupting bladder pain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Alexander JK, Cox GM, Tian JB, Zha AM, Wei P, Kigerl KA, et al.. Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress. Experimental neurology. 2012;236(2):351–362. doi: 10.1016/j.expneurol.2012.04.018 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

