Effects of host extinction and vector preferences on vector-borne disease risk in phylogenetically structured host-hector communities
- PMID: 34424937
- PMCID: PMC8382198
- DOI: 10.1371/journal.pone.0256456
Effects of host extinction and vector preferences on vector-borne disease risk in phylogenetically structured host-hector communities
Abstract
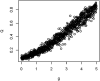
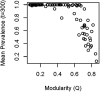
Anthropogenic disturbance impacts the phylogenetic composition and diversity of ecological communities. While changes in diversity are known to dramatically change species interactions and alter disease dynamics, the effects of phylogenetic changes in host and vector communities on disease have been relatively poorly studied. Using a theoretical model, we investigated how phylogeny and extinction influence network structural characteristics relevant to disease transmission in disturbed environments. We modelled a multi-host, multi-vector community as a bipartite ecological network, where nodes represent host and vector species and edges represent connections among them through vector feeding, and we simulated vector preferences and threat status on host and parasite phylogenies. We then simulated loss of hosts, including phylogenetically clustered losses, to investigate how extinction influences network structure. We compared effects of phylogeny and extinction to those of host specificity, which we predicted to strongly increase network modularity and reduce disease prevalence. The simulations revealed that extinction often increased modularity, with higher modularity as species loss increased, although not as much as increasing host specificity did. These results suggest that extinction itself, all else being equal, may reduce disease prevalence in disturbed communities. However, in real communities, systematic patterns in species loss (e.g. favoring high competence species) or changes in abundance may counteract these effects. Unexpectedly, we found that effects of phylogenetic signal in host and vector traits were relatively weak, and only important when phylogenetic signal of host and vector traits were similar, or when these traits both varied.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

