Dorsal Root Ganglion Stimulation for Chronic Pain: Hypothesized Mechanisms of Action
- PMID: 34425252
- PMCID: PMC8943693
- DOI: 10.1016/j.jpain.2021.07.008
Dorsal Root Ganglion Stimulation for Chronic Pain: Hypothesized Mechanisms of Action
Abstract
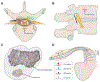
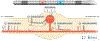
Dorsal root ganglion stimulation (DRGS) is a neuromodulation therapy for chronic pain that is refractory to conventional medical management. Currently, the mechanisms of action of DRGS-induced pain relief are unknown, precluding both our understanding of why DRGS fails to provide pain relief to some patients and the design of neurostimulation technologies that directly target these mechanisms to maximize pain relief in all patients. Due to the heterogeneity of sensory neurons in the dorsal root ganglion (DRG), the analgesic mechanisms could be attributed to the modulation of one or many cell types within the DRG and the numerous brain regions that process sensory information. Here, we summarize the leading hypotheses of the mechanisms of DRGS-induced analgesia, and propose areas of future study that will be vital to improving the clinical implementation of DRGS. PERSPECTIVE: This article synthesizes the evidence supporting the current hypotheses of the mechanisms of action of DRGS for chronic pain and suggests avenues for future interdisciplinary research which will be critical to fully elucidate the analgesic mechanisms of the therapy.
Keywords: Dorsal root ganglion stimulation; chronic pain; electric stimulation; implanted neurostimulators; neuropathic pain.
Copyright © 2021 United States Association for the Study of Pain, Inc. Published by Elsevier Inc. All rights reserved.
Figures
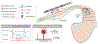
References
-
- Amir R, Liu CN, Kocsis JDK, Devor M: Oscillatory mechanism in primary sensory neurones. Brain 125:421–35, 2002. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

