Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial
- PMID: 34425471
- PMCID: PMC8285932
- DOI: 10.1016/j.phymed.2021.153671
Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial
Abstract
Objective: To evaluate the efficacy and safety of Hua Shi Bai Du Granule (Q-14) plus standard care compared with standard care alone in adults with coronavirus disease (COVID-19).
Study design: A single-center, open-label, randomized controlled trial.
Setting: Wuhan Jinyintan Hospital, Wuhan, China, February 27 to March 27, 2020.
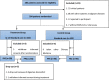
Participants: A total of 204 patients with laboratory-confirmed COVID-19 were randomized into the treatment group and control group, consisting of 102 patients in each group.
Interventions: In the treatment group, Q-14 was administered at 10 g (granules) twice daily for 14 days, plus standard care. In the control group, patients were provided standard care alone for 14 days.
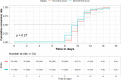
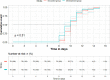
Main outcome measure: The primary outcome was the conversion time for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral assay. Adverse events were analyzed in the safety population.
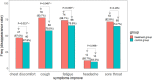
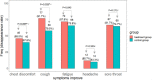
Results: Among the 204 patients, 195 were analyzed according to the intention-to-treat principle. A total of 149 patients (71 vs. 78 in the treatment and control groups, respectively) tested negative via the SARS-CoV-2 viral assay. There was no statistical significance in the conversion time between the treatment group and control group (Full analysis set: Median [interquartile range]: 10.00 [9.00-11.00] vs. 10.00 [9.00-11.00]; Mean rank: 67.92 vs. 81.44; P = 0.051). The recovery time for fever was shorter in the treatment group than in the control group. The disappearance rate of symptoms like cough, fatigue, and chest discomfort was significantly higher in the treatment group. In chest computed tomography (CT) examinations, the overall evaluation of chest CT examination after treatment compared with baseline showed that more patients improved in the treatment group. There were no significant differences in the other outcomes.
Conclusion: The combination of Q-14 and standard care for COVID-19 was useful for the improvement of symptoms (such as fever, cough, fatigue, and chest discomfort), but did not result in a significantly higher probability of negative conversion in the SARS-CoV-2 viral assay. No serious adverse events were observed.
Trial registration: ChiCTR2000030288.
Keywords: COVID-19; Chinese medicine; Hua Shi Bai Du Granule; Randomized Controlled Trial; efficacy; safety.
Copyright © 2021. Published by Elsevier GmbH.
Conflict of interest statement
None
Figures


References
-
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q., 2020. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ368, m1091. doi: 10.1136/bmj.m1091. - PMC - PubMed
-
- Elhadi M, Msherghi A, Elgzairi M, Alhashimi A, Bouhuwaish A, Biala M, Abuelmeda S, Khel S, Khaled A, Alsoufi A, Elmabrouk A, Alshiteewi FB, Alhadi B, Alhaddad S, Gaffaz R, Elmabrouk O, Hamed T.B., Alameen H, Zaid A, Elhadi A, Albakoush A. Psychological status of healthcare workers during the civil war and COVID-19 pandemic: A cross-sectional study. J. Psychosom. Res. 2020;137 doi: 10.1016/j.jpsychores.2020.110221. - DOI - PMC - PubMed
-
- Hu K, Guan W.J., Bi Y, Zhang W, Li L, Zhang B, Liu Q, Song Y, Li X, Duan Z, Zheng Q, Yang Z, Liang J, Han M, Ruan L, Wu C, Zhang Y, Jia ZH, Zhong NS. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

