Organelle Dynamics in Apicomplexan Parasites
- PMID: 34425697
- PMCID: PMC8406264
- DOI: 10.1128/mBio.01409-21
Organelle Dynamics in Apicomplexan Parasites
Abstract
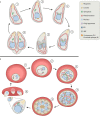
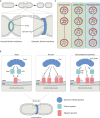
Apicomplexan parasites, such as Toxoplasma gondii and Plasmodium falciparum, are the cause of many important human and animal diseases. While T. gondii tachyzoites replicate through endodyogeny, during which two daughter cells are formed within the parental cell, P. falciparum replicates through schizogony, where up to 32 parasites are formed in a single infected red blood cell and even thousands of daughter cells during mosquito- or liver-stage development. These processes require a tightly orchestrated division and distribution over the daughter parasites of one-per-cell organelles such as the mitochondrion and apicoplast. Although proper organelle segregation is highly essential, the molecular mechanism and the key proteins involved remain largely unknown. In this review, we describe organelle dynamics during cell division in T. gondii and P. falciparum, summarize the current understanding of the molecular mechanisms underlying organelle fission in these parasites, and introduce candidate fission proteins.
Keywords: Plasmodium; Toxoplasma; dynamins; mitochondrion; organelle segregation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

