Describing global pediatric RSV disease at intensive care units in GAVI-eligible countries using molecular point-of-care diagnostics: the RSV GOLD-III study protocol
- PMID: 34425773
- PMCID: PMC8380869
- DOI: 10.1186/s12879-021-06544-3
Describing global pediatric RSV disease at intensive care units in GAVI-eligible countries using molecular point-of-care diagnostics: the RSV GOLD-III study protocol
Erratum in
-
Correction to: Describing global pediatric RSV disease at intensive care units in GAVI-eligible countries using molecular point-of-care diagnostics: the RSV GOLD-III study protocol.BMC Infect Dis. 2021 Sep 16;21(1):962. doi: 10.1186/s12879-021-06671-x. BMC Infect Dis. 2021. PMID: 34530766 Free PMC article. No abstract available.
Abstract
Background: Respiratory syncytial virus (RSV) infection is an important cause of hospitalization and death in young children. The majority of deaths (99%) occur in low- and lower-middle-income countries (LMICs). Vaccines against RSV infection are underway. To obtain access to RSV interventions, LMICs depend on support from Gavi, the Vaccine Alliance. To identify future vaccine target populations, information on children with severe RSV infection is required. However, there is a lack of individual patient-level clinical data on instances of life-threatening RSV infection in LMICs. The RSV GOLD III-ICU Network study aims to describe clinical, demographic and socioeconomic characteristics of children with life-threatening RSV infection in Gavi-eligible countries.
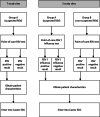
Methods: The RSV GOLD-III-ICU Network study is an international, prospective, observational multicenter study and will be conducted in 10 Gavi-eligible countries at pediatric intensive care units and high-dependency units (PICUs/HDUs) during local viral respiratory seasons for 2 years. Children younger than 2 years of age with respiratory symptoms fulfilling the World Health Organization (WHO) "extended severe acute respiratory infection (SARI)" case definition will be tested for RSV using a molecular point-of-care (POC) diagnostic device. Patient characteristics will be collected through a questionnaire. Mortality rates of children admitted to the PICU and/or HDU will be calculated.
Discussion: This multicenter descriptive study will provide a better understanding of the characteristics and mortality rates of children younger than 2 years with RSV infection admitted to the PICU/HDU in LMICs. These results will contribute to knowledge on global disease burden and awareness of RSV and will directly guide decision makers in their efforts to implement future RSV prevention strategies.
Trial registration number: NL9519, May 27, 2021.
Keywords: Awareness; Burden; Children; Lower-middle-income countries; Pediatric intensive care unit; Respiratory syncytial virus; Study design.
© 2021. The Author(s).
Conflict of interest statement
LJB has regular interaction with pharmaceutical and other industrial partners. He has not received personal fees or other personal benefits. UMCU has received major funding (> €100,000 per industrial partner) for investigator-initiated studies from AbbVie, MedImmune, Janssen, the Bill and Melinda Gates Foundation, Nutricia (Danone) and MeMed Diagnostics. UMCU has received major cash or in kind funding as part of the public private partnership IMI-funded RESCEU project from GSK, Novavax, Janssen, AstraZeneca, Pfizer and Sanofi. UMCU has received major funding by Julius Clinical for participating in the INFORM study sponsored by MedImmune. UMCU has received minor funding for participation in trials by Regeneron and Janssen from 2015 to 2017 (total annual estimate less than €20,000). UMCU received minor funding for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, Novavax, Pfizer, Janssen (total annual estimate less than €20,000). LJB is the founding chairman of the ReSViNET Foundation. NIM has regular interaction with pharmaceutical and other industrial partners. She has not received personal fees or other personal benefits. HN reports grants from Innovative Medicines Initiative and National Institute of Health Research; personal fees and grants from WHO and Sanofi; and personal fees from the Bill & Melinda Gates Foundation, Janssen, Abbvie, and Reviral.GvT is involved in public private partnerships (EU Innovative Medicines Initiative consortia). She has not received personal fees or other personal benefits.
Figures
References
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
-
- PATH. RSV Vaccine and mAb Snapshot 2020. Available from: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/. Accessed 22 Jun 2021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

