Paradoxical effects of cigarette smoke and COPD on SARS-CoV-2 infection and disease
- PMID: 34425811
- PMCID: PMC8381712
- DOI: 10.1186/s12890-021-01639-8
Paradoxical effects of cigarette smoke and COPD on SARS-CoV-2 infection and disease
Abstract
Background: How cigarette smoke (CS) and chronic obstructive pulmonary disease (COPD) affect severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection and severity is controversial. We investigated the effects of COPD and CS on the expression of SARS-CoV-2 entry receptor ACE2 in vivo in COPD patients and controls and in CS-exposed mice, and the effects of CS on SARS-CoV-2 infection in human bronchial epithelial cells in vitro.
Methods: We quantified: (1) pulmonary ACE2 protein levels by immunostaining and ELISA, and both ACE2 and/or TMPRSS2 mRNA levels by RT-qPCR in two independent human cohorts; and (2) pulmonary ACE2 protein levels by immunostaining and ELISA in C57BL/6 WT mice exposed to air or CS for up to 6 months. The effects of CS exposure on SARS-CoV-2 infection were evaluated after in vitro infection of Calu-3 cells and differentiated human bronchial epithelial cells (HBECs), respectively.
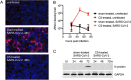
Results: ACE2 protein and mRNA levels were decreased in peripheral airways from COPD patients versus controls but similar in central airways. Mice exposed to CS had decreased ACE2 protein levels in their bronchial and alveolar epithelia versus air-exposed mice. CS treatment decreased viral replication in Calu-3 cells, as determined by immunofluorescence staining for replicative double-stranded RNA (dsRNA) and western blot for viral N protein. Acute CS exposure decreased in vitro SARS-CoV-2 replication in HBECs, as determined by plaque assay and RT-qPCR.
Conclusions: ACE2 levels were decreased in both bronchial and alveolar epithelial cells from COPD patients versus controls, and from CS-exposed versus air-exposed mice. CS-pre-exposure potently inhibited SARS-CoV-2 replication in vitro. These findings urge to investigate further the controversial effects of CS and COPD on SARS-CoV-2 infection.
© 2021. The Author(s).
Conflict of interest statement
M. Contoli has received personal fees from Chiesi, AstraZeneca, Boehringer-Ingelheim, Alk-Abello, GSK, Novartis, Zambon, and scientific grants from Chiesi and University of Ferrara, Italy. A. Papi: Board membership, consultancy, payment for lectures, grants for research, travel expenses reimbursement from GSK, AZ, Boehringer Ingelheim, Chiesi Farmaceutici, TEVA, Mundipharma, Zambon, Novartis, Menarini, Sanofi,Roche, Edmondpharma, Fondazione Maugeri, Fondazione Chiesi. None of the other authors have competing conflicts of interest to declare.
Figures



Update of
-
Paradoxical effects of cigarette smoke and COPD on SARS-CoV2 infection and disease.bioRxiv [Preprint]. 2020 Dec 7:2020.12.07.413252. doi: 10.1101/2020.12.07.413252. bioRxiv. 2020. Update in: BMC Pulm Med. 2021 Aug 23;21(1):275. doi: 10.1186/s12890-021-01639-8. PMID: 33330864 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R00 DK103126/DK/NIDDK NIH HHS/United States
- HL130045/HL/NHLBI NIH HHS/United States
- HL091889/HL/NHLBI NIH HHS/United States
- P30 ES006694/ES/NIEHS NIH HHS/United States
- U01 AI126614/AI/NIAID NIH HHS/United States
- HL098112/HL/NHLBI NIH HHS/United States
- R01 GM136853/GM/NIGMS NIH HHS/United States
- U10 HL098112/HL/NHLBI NIH HHS/United States
- R01 HL091889/HL/NHLBI NIH HHS/United States
- R01 HL142769/HL/NHLBI NIH HHS/United States
- ES006614/ES/NIEHS NIH HHS/United States
- U01 HL130045/HL/NHLBI NIH HHS/United States
- UH3 OD023282/OD/NIH HHS/United States
- HL056177/HL/NHLBI NIH HHS/United States
- HL139054/HL/NHLBI NIH HHS/United States
- T32 CA009213/CA/NCI NIH HHS/United States
- HL132523/HL/NHLBI NIH HHS/United States
- UG3 OD023282/OD/NIH HHS/United States
- DK103126/DK/NIDDK NIH HHS/United States
- HL149744/HL/NHLBI NIH HHS/United States
- K99 DK103126/DK/NIDDK NIH HHS/United States
- R01 HL149744/HL/NHLBI NIH HHS/United States
- R01 HL132523/HL/NHLBI NIH HHS/United States
- GM136853/GM/NIGMS NIH HHS/United States
- UG1 HL139054/HL/NHLBI NIH HHS/United States
- R41 CA271967/CA/NCI NIH HHS/United States
- R01 HL056177/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

