Acute Kidney Injury in Severe COVID-19 Has Similarities to Sepsis-Associated Kidney Injury: A Multi-Omics Study
- PMID: 34425963
- PMCID: PMC8279954
- DOI: 10.1016/j.mayocp.2021.07.001
Acute Kidney Injury in Severe COVID-19 Has Similarities to Sepsis-Associated Kidney Injury: A Multi-Omics Study
Abstract
Objective: To compare coronavirus disease 2019 (COVID-19) acute kidney injury (AKI) to sepsis-AKI (S-AKI). The morphology and transcriptomic and proteomic characteristics of autopsy kidneys were analyzed.
Patients and methods: Individuals 18 years of age and older who died from COVID-19 and had an autopsy performed at Mayo Clinic between April 2020 to October 2020 were included. Morphological evaluation of the kidneys of 17 individuals with COVID-19 was performed. In a subset of seven COVID-19 cases with postmortem interval of less than or equal to 20 hours, ultrastructural and molecular characteristics (targeted transcriptome and proteomics analyses of tubulointerstitium) were evaluated. Molecular characteristics were compared with archived cases of S-AKI and nonsepsis causes of AKI.
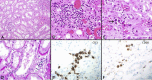
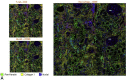
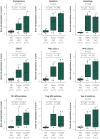
Results: The spectrum of COVID-19 renal pathology included macrophage-dominant microvascular inflammation (glomerulitis and peritubular capillaritis), vascular dysfunction (peritubular capillary congestion and endothelial injury), and tubular injury with ultrastructural evidence of mitochondrial damage. Investigation of the spatial architecture using a novel imaging mass cytometry revealed enrichment of CD3+CD4+ T cells in close proximity to antigen-presenting cells, and macrophage-enriched glomerular and interstitial infiltrates, suggesting an innate and adaptive immune tissue response. Coronavirus disease 2019 AKI and S-AKI, as compared to nonseptic AKI, had an enrichment of transcriptional pathways involved in inflammation (apoptosis, autophagy, major histocompatibility complex class I and II, and type 1 T helper cell differentiation). Proteomic pathway analysis showed that COVID-19 AKI and to a lesser extent S-AKI were enriched in necroptosis and sirtuin-signaling pathways, both involved in regulatory response to inflammation. Upregulation of the ceramide-signaling pathway and downregulation of oxidative phosphorylation in COVID-19 AKI were noted.
Conclusion: This data highlights the similarities between S-AKI and COVID-19 AKI and suggests that mitochondrial dysfunction may play a pivotal role in COVID-19 AKI. This data may allow the development of novel diagnostic and therapeutic targets.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Comparison of renal histopathology and gene expression profiles between severe COVID-19 and bacterial sepsis in critically ill patients.Crit Care. 2021 Jun 10;25(1):202. doi: 10.1186/s13054-021-03631-4. Crit Care. 2021. PMID: 34112226 Free PMC article.
-
Covid-19 and kidney injury: Pathophysiology and molecular mechanisms.Rev Med Virol. 2021 May;31(3):e2176. doi: 10.1002/rmv.2176. Epub 2020 Oct 6. Rev Med Virol. 2021. PMID: 33022818 Free PMC article. Review.
-
Postmortem Kidney Pathology Findings in Patients with COVID-19.J Am Soc Nephrol. 2020 Sep;31(9):2158-2167. doi: 10.1681/ASN.2020050744. Epub 2020 Jul 29. J Am Soc Nephrol. 2020. PMID: 32727719 Free PMC article.
-
Pathophysiology of COVID-19-associated acute kidney injury.Nat Rev Nephrol. 2021 Nov;17(11):751-764. doi: 10.1038/s41581-021-00452-0. Epub 2021 Jul 5. Nat Rev Nephrol. 2021. PMID: 34226718 Free PMC article. Review.
-
Single-cell RNA sequencing analysis of human kidney reveals the presence of ACE2 receptor: A potential pathway of COVID-19 infection.Mol Genet Genomic Med. 2020 Oct;8(10):e1442. doi: 10.1002/mgg3.1442. Epub 2020 Aug 3. Mol Genet Genomic Med. 2020. PMID: 32744436 Free PMC article.
Cited by
-
The Fight Against Severe COVID-19: Can Parasitic Worms Contribute?Front Immunol. 2022 Feb 11;13:849465. doi: 10.3389/fimmu.2022.849465. eCollection 2022. Front Immunol. 2022. PMID: 35222441 Free PMC article. No abstract available.
-
Epigenetic dysregulation of autophagy in sepsis-induced acute kidney injury: the underlying mechanisms for renoprotection.Front Immunol. 2023 May 5;14:1180866. doi: 10.3389/fimmu.2023.1180866. eCollection 2023. Front Immunol. 2023. PMID: 37215112 Free PMC article. Review.
-
Proteomic Characterization of Acute Kidney Injury in Patients Hospitalized with SARS-CoV2 Infection.medRxiv [Preprint]. 2022 Aug 29:2021.12.09.21267548. doi: 10.1101/2021.12.09.21267548. medRxiv. 2022. Update in: Commun Med (Lond). 2023 Jun 12;3(1):81. doi: 10.1038/s43856-023-00307-8. PMID: 36093350 Free PMC article. Updated. Preprint.
-
COVID-19 and Kidney: The Importance of Follow-Up and Long-Term Screening.Life (Basel). 2023 Oct 30;13(11):2137. doi: 10.3390/life13112137. Life (Basel). 2023. PMID: 38004277 Free PMC article. Review.
-
Safety of Citrate Anticoagulation in CKRT: Monocentric Experience of a Dynamic Protocol of Calcium Monitoring.J Clin Med. 2023 Aug 10;12(16):5201. doi: 10.3390/jcm12165201. J Clin Med. 2023. PMID: 37629242 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

