Could live attenuated vaccines better control COVID-19?
- PMID: 34426024
- PMCID: PMC8354792
- DOI: 10.1016/j.vaccine.2021.08.018
Could live attenuated vaccines better control COVID-19?
Abstract
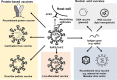
In an effort to control the COVID-19 pandemic, large-scale vaccination is being implemented in various countries using anti-SARS-CoV-2 vaccines based on mRNAs, adenovirus vectors, and inactivated viruses. However, there are concerns regarding adverse effects, such as the induction of fever attributed to mRNA vaccines and pre-existing immunity against adenovirus vectored vaccines or their possible involvement in the development of thrombosis. The induction of antibodies against the adenovirus vector itself constitutes another hindrance, rendering boosting vaccinations ineffective. Additionally, it has been questioned whether inactivated vaccines that predominantly induce humoral immunity are effective against newly arising variants, as some isolated strains were found to be resistant to the serum from COVID-19-recovered patients. Although the number of vaccinated people is steadily increasing on a global scale, it is still necessary to develop vaccines to address the difficulties and concerns mentioned above. Among the various vaccine modalities, live attenuated vaccines have been considered the most effective, since they closely replicate a natural infection without the burden of the disease. In our attempt to provide an additional option to the repertoire of COVID-19 vaccines, we succeeded in isolating temperature-sensitive strains with unique phenotypes that could serve as seeds for a live attenuated vaccine. In this review article, we summarize the characteristics of the currently approved SARS-CoV-2 vaccines and discuss their advantages and disadvantages. In particular, we focus on the novel temperature-sensitive variants of SARS-CoV-2 that we have recently isolated, and their potential application as live-attenuated vaccines. Based on a thorough evaluation of the different vaccine modalities, we argue that it is important to optimize usage not only based on efficacy, but also on the phases of the pandemic. Our findings can be used to inform vaccination practices and improve global recovery from the COVID-19 pandemic.
Keywords: COVID-19; Live attenuated vaccine; SARS-CoV-2; Vaccine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Current scenario of COVID-19 vaccinations and immune response along with antibody titer in vaccinated inhabitants of different countries.Int Immunopharmacol. 2021 Oct;99:108050. doi: 10.1016/j.intimp.2021.108050. Epub 2021 Aug 6. Int Immunopharmacol. 2021. PMID: 34426120 Free PMC article. Review.
-
Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters.Nat Microbiol. 2023 May;8(5):860-874. doi: 10.1038/s41564-023-01352-8. Epub 2023 Apr 3. Nat Microbiol. 2023. PMID: 37012419 Free PMC article.
-
COVID-19 vaccines: concerns beyond protective efficacy and safety.Expert Rev Vaccines. 2021 Aug;20(8):1013-1025. doi: 10.1080/14760584.2021.1949293. Epub 2021 Jul 5. Expert Rev Vaccines. 2021. PMID: 34180347 Review.
-
[Technical guidelines for seasonal influenza vaccination in China (2022-2023)].Zhonghua Yu Fang Yi Xue Za Zhi. 2022 Oct 6;56(10):1356-1386. doi: 10.3760/cma.j.cn112150-20220825-00840. Zhonghua Yu Fang Yi Xue Za Zhi. 2022. PMID: 36274602 Chinese.
-
COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions.Front Cell Infect Microbiol. 2021 Sep 10;11:690621. doi: 10.3389/fcimb.2021.690621. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34568087 Free PMC article. Review.
Cited by
-
A recombination-resistant genome for live attenuated and stable PEDV vaccines by engineering the transcriptional regulatory sequences.J Virol. 2023 Dec 21;97(12):e0119323. doi: 10.1128/jvi.01193-23. Epub 2023 Nov 16. J Virol. 2023. PMID: 37971221 Free PMC article.
-
Recent advances in the vaccine development for the prophylaxis of SARS Covid-19.Int Immunopharmacol. 2022 Oct;111:109175. doi: 10.1016/j.intimp.2022.109175. Epub 2022 Aug 17. Int Immunopharmacol. 2022. PMID: 35994853 Free PMC article. Review.
-
Indirect Dispersion of SARS-CoV-2 Live-Attenuated Vaccine and Its Contribution to Herd Immunity.Vaccines (Basel). 2023 Mar 14;11(3):655. doi: 10.3390/vaccines11030655. Vaccines (Basel). 2023. PMID: 36992239 Free PMC article. Review.
-
From Immunogen to COVID-19 vaccines: Prospects for the post-pandemic era.Biomed Pharmacother. 2023 Feb;158:114208. doi: 10.1016/j.biopha.2022.114208. Epub 2023 Jan 2. Biomed Pharmacother. 2023. PMID: 36800265 Free PMC article. Review.
-
Development of variant-proof severe acute respiratory syndrome coronavirus 2, pan-sarbecovirus, and pan-β-coronavirus vaccines.J Med Virol. 2023 Jan;95(1):e28172. doi: 10.1002/jmv.28172. Epub 2022 Oct 6. J Med Virol. 2023. PMID: 36161303 Free PMC article. Review.
References
-
- Center C-M-JHCR.
-
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

