Establishment of Patient-Derived Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumor Models for Predicting Therapeutic Response
- PMID: 34426440
- PMCID: PMC8738129
- DOI: 10.1158/1078-0432.CCR-21-2092
Establishment of Patient-Derived Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumor Models for Predicting Therapeutic Response
Abstract
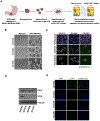
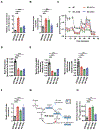
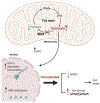
Purpose: Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the gastrointestinal tract, with mutant succinate dehydrogenase (SDH) subunits (A-D) comprising less than 7.5% (i.e., 150-200/year) of new cases annually in the United States. Contrary to GISTs harboring KIT or PDGFRA mutations, SDH-mutant GISTs affect adolescents/young adults, often metastasize, and are frequently resistant to tyrosine kinase inhibitors (TKI). Lack of human models for any SDH-mutant tumors, including GIST, has limited molecular characterization and drug discovery.
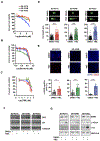
Experimental design: We describe methods for establishing novel patient-derived SDH-mutant (mSDH) GIST models and interrogated the efficacy of temozolomide on these tumor models in vitro and in clinical trials of patients with mSDH GIST.
Results: Molecular and metabolic characterization of our patient-derived mSDH GIST models revealed that these models recapitulate the transcriptional and metabolic hallmarks of parent tumors and SDH deficiency. We further demonstrate that temozolomide elicits DNA damage and apoptosis in our mSDH GIST models. Translating our in vitro discovery to the clinic, a cohort of patients with SDH-mutant GIST treated with temozolomide (n = 5) demonstrated a 40% objective response rate and 100% disease control rate, suggesting that temozolomide represents a promising therapy for this subset of GIST.
Conclusions: We report the first methods to establish patient-derived mSDH tumor models, which can be readily employed for understanding patient-specific tumor biology and treatment strategies. We also demonstrate that temozolomide is effective in patients with mSDH GIST who are refractory to existing chemotherapeutic drugs (namely, TKIs) in clinic for GISTs, bringing a promising treatment option for these patients to clinic.See related commentary by Blakely et al., p. 3.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures


Comment in
-
Taming the Wild-Type Gastrointestinal Stromal Tumor: Improved Tissue Culture.Clin Cancer Res. 2022 Jan 1;28(1):3-4. doi: 10.1158/1078-0432.CCR-21-3409. Epub 2021 Oct 28. Clin Cancer Res. 2022. PMID: 34711630 Free PMC article.
References
-
- Gebreyohannes YK, Wozniak A, Zhai ME, Wellens J, Cornillie J, Vanleeuw U, et al. Robust Activity of Avapritinib, Potent and Highly Selective Inhibitor of Mutated KIT, in Patient-derived Xenograft Models of Gastrointestinal Stromal Tumors. Clin Cancer Res 2019;25(2):609–18 doi 10.1158/1078-0432.CCR-18-1858. - DOI - PubMed
-
- Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet 2002;108(2):132–9. - PubMed
-
- Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 2008;16(1):79–88 doi 10.1038/sj.ejhg.5201904. - DOI - PubMed
-
- Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov 2013;3(6):648–57 doi 10.1158/2159-8290.CD-13-0092. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

