Phase I Study of 2- or 3-Week Dosing of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, Monotherapy in Patients with Advanced Non-Small Cell Lung Carcinoma
- PMID: 34426443
- PMCID: PMC9401525
- DOI: 10.1158/1078-0432.CCR-21-0765
Phase I Study of 2- or 3-Week Dosing of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, Monotherapy in Patients with Advanced Non-Small Cell Lung Carcinoma
Abstract
Purpose: Telisotuzumab vedotin (Teliso-V) is an anti-c-Met-directed antibody-drug conjugate. Here, we present safety and efficacy data from a phase I/Ib study of Teliso-V monotherapy evaluated in once every 2 weeks/once every 3 weeks schedules in patients with non-small cell lung cancer (NSCLC).
Patients and methods: During dose escalation, patients received Teliso-V monotherapy intravenously once every 3 weeks (0.15-3.3 mg/kg) or once every 2 weeks (1.6-2.2 mg/kg). The dose-expansion phase enrolled patients with NSCLC and c-Met H-score ≥150 (c-Met+) or MET amplification/exon 14 skipping mutations. Safety, pharmacokinetics, and efficacy were assessed. Herein, the analysis of patients receiving ≥1.6 mg/kg once every 2 weeks or ≥2.4 mg/kg once every 3 weeks Teliso-V is reported.
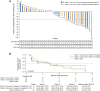
Results: Fifty-two patients with NSCLC were enrolled and received ≥1.6 mg/kg Teliso-V once every 2 weeks (n = 28) or ≥2.4 mg/kg Teliso-V once every 3 weeks (n = 24). The most common adverse events were fatigue (54%), peripheral neuropathy (42%), and nausea (38%). No dose-limiting toxicities were observed for Teliso-V once every 2 weeks and once every 3 weeks up to 2.2 and 2.7 mg/kg, respectively. The recommended phase II dose was established at 1.9 mg/kg once every 2 weeks and 2.7 mg/kg once every 3 weeks on the basis of overall safety and pharmacokinetics. Forty of 52 patients were c-Met+ (33 nonsquamous, 6 squamous, 1 mixed histology) and were included in the efficacy-evaluable population. Of those, 9 (23%) had objective responses with median duration of response of 8.7 months; median progression-free survival was 5.2 months.
Conclusions: Teliso-V monotherapy was tolerated and showed antitumor activity in c-Met+ NSCLC. On the basis of overall safety, pharmacokinetics, and efficacy outcomes, 1.9 mg/kg Teliso-V once every 2 weeks and 2.7 mg/kg once every 3 weeks schedules were selected for further clinical development.
Trial registration: ClinicalTrials.gov NCT02099058.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev 2003;22:309–25. - PubMed
-
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89–103. - PubMed
-
- Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005;65:1479–88. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

