Clock proteins and training modify exercise capacity in a daytime-dependent manner
- PMID: 34426495
- PMCID: PMC8536342
- DOI: 10.1073/pnas.2101115118
Clock proteins and training modify exercise capacity in a daytime-dependent manner
Abstract
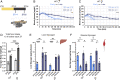
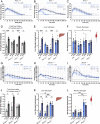
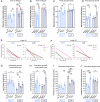
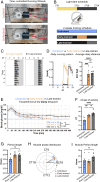
Exercise and circadian biology are closely intertwined with physiology and metabolism, yet the functional interaction between circadian clocks and exercise capacity is only partially characterized. Here, we tested different clock mutant mouse models to examine the effect of the circadian clock and clock proteins, namely PERIODs and BMAL1, on exercise capacity. We found that daytime variance in endurance exercise capacity is circadian clock controlled. Unlike wild-type mice, which outperform in the late compared with the early part of their active phase, PERIODs- and BMAL1-null mice do not show daytime variance in exercise capacity. It appears that BMAL1 impairs and PERIODs enhance exercise capacity in a daytime-dependent manner. An analysis of liver and muscle glycogen stores as well as muscle lipid utilization suggested that these daytime effects mostly relate to liver glycogen levels and correspond to the animals' feeding behavior. Furthermore, given that exercise capacity responds to training, we tested the effect of training at different times of the day and found that training in the late compared with the early part of the active phase improves exercise performance. Overall, our findings suggest that clock proteins shape exercise capacity in a daytime-dependent manner through changes in liver glycogen levels, likely due to their effect on animals' feeding behavior.
Keywords: circadian clocks; exercise; glycogen; metabolism; training.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Gabriel B. M., Zierath J. R., The limits of exercise physiology: From performance to health. Cell Metab. 25, 1000–1011 (2017). - PubMed
-
- Hawley J. A., Hargreaves M., Joyner M. J., Zierath J. R., Integrative biology of exercise. Cell 159, 738–749 (2014). - PubMed
-
- Stephenson E. J., Smiles W., Hawley J. A., The relationship between exercise, nutrition and type 2 diabetes. Med. Sport Sci. 60, 1–10 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

