Regulatory T cells function in established systemic inflammation and reverse fatal autoimmunity
- PMID: 34426690
- PMCID: PMC9341271
- DOI: 10.1038/s41590-021-01001-4
Regulatory T cells function in established systemic inflammation and reverse fatal autoimmunity
Abstract
The immunosuppressive function of regulatory T (Treg) cells is dependent on continuous expression of the transcription factor Foxp3. Foxp3 loss of function or induced ablation of Treg cells results in a fatal autoimmune disease featuring all known types of inflammatory responses with every manifestation stemming from Treg cell paucity, highlighting a vital function of Treg cells in preventing fatal autoimmune inflammation. However, a major question remains whether Treg cells can persist and effectively exert their function in a disease state, where a broad spectrum of inflammatory mediators can either inactivate Treg cells or render innate and adaptive pro-inflammatory effector cells insensitive to suppression. By reinstating Foxp3 protein expression and suppressor function in cells expressing a reversible Foxp3 null allele in severely diseased mice, we found that the resulting single pool of rescued Treg cells normalized immune activation, quelled severe tissue inflammation, reversed fatal autoimmune disease and provided long-term protection against them. Thus, Treg cells are functional in settings of established broad-spectrum systemic inflammation and are capable of affording sustained reset of immune homeostasis.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interest:
A.Y.R. is a co-founder and SAB member of and holds stock options in Vedanta Bioscience and Sonoma Biotherapeitics. No other authors declare any conflict of interest.
Figures



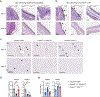




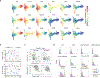







Comment in
-
Restoring control over autoimmunity by inducing Foxp3.Nat Immunol. 2021 Sep;22(9):1080-1082. doi: 10.1038/s41590-021-01008-x. Nat Immunol. 2021. PMID: 34426689 No abstract available.
-
Tregs and inflammation-bring it on!Sci Immunol. 2021 Oct;6(64):eabm5116. doi: 10.1126/sciimmunol.abm5116. Epub 2021 Oct 1. Sci Immunol. 2021. PMID: 34597122
-
Restoration of established systemic inflammation and autoimmunity by Foxp3+ regulatory T cells.Cell Mol Immunol. 2022 Feb;19(2):133-135. doi: 10.1038/s41423-021-00831-4. Epub 2022 Jan 7. Cell Mol Immunol. 2022. PMID: 34992284 Free PMC article. No abstract available.
References
-
- Kanangat S et al. Disease in the scurfy (sf) mouse is associated with overexpression of cytokine genes. Eur J Immunol 26, 161–165 (1996). - PubMed
-
- Chatila TA Role of regulatory T cells in human diseases. J Allergy Clin Immunol 116, 949–959; quiz 960 (2005). - PubMed
-
- Khattri R, Cox T, Yasayko SA & Ramsdell F An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 4, 337–342 (2003). - PubMed
-
- Fontenot JD, Gavin MA & Rudensky AY Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4, 330–336 (2003). - PubMed
Online Methods References
-
- Mombaerts P et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature 360, 225–231 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

