Lentivirus- or AAV-mediated gene therapy interventions in ischemic stroke: A systematic review of preclinical in vivo studies
- PMID: 34427147
- PMCID: PMC8795232
- DOI: 10.1177/0271678X211039997
Lentivirus- or AAV-mediated gene therapy interventions in ischemic stroke: A systematic review of preclinical in vivo studies
Abstract
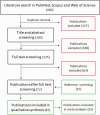
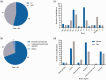
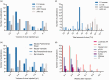
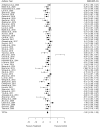
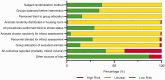
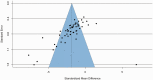
Due to the limited therapeutic options after ischemic stroke, gene therapy has emerged as a promising choice, especially with recent advances in viral vector delivery systems. Therefore, we aimed to provide the current state of the art of lentivirus (LV) and adeno-associated virus (AAV) mediated gene interventions in preclinical ischemic stroke models. A systematic analysis including qualitative and quantitative syntheses of studies published until December 2020 was performed. Most of the 87 selected publications used adult male rodents and the preferred stroke model was transient middle cerebral artery occlusion. LV and AAV vectors were equally used for transgene delivery, however loads of AAVs were higher than LVs. Serotypes having broad cell tropism, the use of constitutive promoters, and virus delivery before the stroke induction via stereotaxic injection in the cortex and striatum were preferred in the analyzed studies. The meta-analysis based on infarct volume as the primary outcome confirmed the efficacy of the preclinical interventions. The quality assessment exposed publication bias and setbacks in regard to risks of bias and study relevance. The translational potential could increase by using specific cell targeting, post-stroke interventions, non-invasive systematic delivery, and use of large animals.
Keywords: Adeno-associated virus; gene therapy; infarct; ischemic stroke; lentivirus.
Conflict of interest statement
Figures


References
-
- Nogles TE, Galuska MA. Middle cerebral artery stroke. Treasure Island, FL: StatPearls Publishing, 2020. - PubMed
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, et al.. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke. Stroke 2019; 50: e344–e418. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

