Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies
- PMID: 34427172
- PMCID: PMC8439218
- DOI: 10.1080/22221751.2021.1971569
Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies
Abstract
The unprecedented in recent history global COVID-19 pandemic urged the implementation of all existing vaccine platforms to ensure the availability of the vaccines against COVID-19 to every country in the world. Despite the multitude of high-quality papers describing clinical trials of different vaccine products, basic detailed data on general toxicity, reproductive toxicity, immunogenicity, protective efficacy and durability of immune response in animal models are scarce. Here, we developed a β-propiolactone-inactivated whole virion vaccine CoviVac and assessed its safety, protective efficacy, immunogenicity and stability of the immune response in rodents and non-human primates. The vaccine showed no signs of acute/chronic, reproductive, embryo- and fetotoxicity, or teratogenic effects, as well as no allergenic properties in studied animal species. The vaccine induced stable and robust humoral immune response both in form of specific anti-SARS-CoV-2 IgG and NAbs in mice, Syrian hamsters, and common marmosets. The NAb levels did not decrease significantly over the course of one year. The course of two immunizations protected Syrian hamsters from severe pneumonia upon intranasal challenge with the live virus. Robustness of the vaccine manufacturing process was demonstrated as well. These data encouraged further evaluation of CoviVac in clinical trials.
Keywords: COVID-19; SARS-CoV-2; Vaccine; immunogenicity; inactivated vaccine; neutralizing antibodies; preclinical study; safety.
Conflict of interest statement
Most of the authors work for Chumakov FSC R&D IBP RAS - CoviVac developer and producer.
Figures
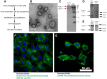

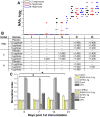
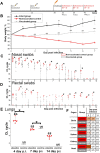
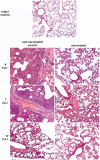


References
-
- WHO COVID-19 Dashboard [Internet]. Geneva: World Health Organization; 2020 [cited 2021 Aug 12]. Available from: https://covid19.who.int/.
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [Internet]. [cited 2021 Mar 27]. Available from: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594....
-
- International Committee on Taxonomy of Viruses (ICTV) [Internet]. [cited 2021 Mar 14]. Available from: https://ictv.global/taxonomy/.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
