Antitumor Necrosis Factor-like Ligand 1A Therapy Targets Tissue Inflammation and Fibrosis Pathways and Reduces Gut Pathobionts in Ulcerative Colitis
- PMID: 34427649
- PMCID: PMC8889296
- DOI: 10.1093/ibd/izab193
Antitumor Necrosis Factor-like Ligand 1A Therapy Targets Tissue Inflammation and Fibrosis Pathways and Reduces Gut Pathobionts in Ulcerative Colitis
Abstract
Background: The first-in-class treatment PF-06480605 targets the tumor necrosis factor-like ligand 1A (TL1A) molecule in humans. Results from the phase 2a TUSCANY trial highlighted the safety and efficacy of PF-06480605 in ulcerative colitis. Preclinical and in vitro models have identified a role for TL1A in both innate and adaptive immune responses, but the mechanisms underlying the efficacy of anti-TL1A treatment in inflammatory bowel disease (IBD) are not known.
Methods: Here, we provide analysis of tissue transcriptomic, peripheral blood proteomic, and fecal metagenomic data from the recently completed phase 2a TUSCANY trial and demonstrate endoscopic improvement post-treatment with PF-06480605 in participants with ulcerative colitis.
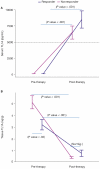
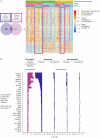
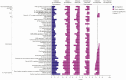
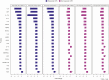
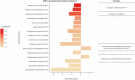
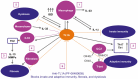
Results: Our results revealed robust TL1A target engagement in colonic tissue and a distinct colonic transcriptional response reflecting a reduction in inflammatory T helper 17 cell, macrophage, and fibrosis pathways in patients with endoscopic improvement. Proteomic analysis of peripheral blood revealed a corresponding decrease in inflammatory T-cell cytokines. Finally, microbiome analysis showed significant changes in IBD-associated pathobionts, Streptococcus salivarius, S. parasanguinis, and Haemophilus parainfluenzae post-therapy.
Conclusions: The ability of PF-06480605 to engage and inhibit colonic TL1A, targeting inflammatory T cell and fibrosis pathways, provides the first-in-human mechanistic data to guide anti-TL1A therapy for the treatment of IBD.
Keywords: TL1A inhibition; inflammatory bowel disease; transcriptional response; ulcerative colitis.
© 2021 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
M.H.Z., Z.Y., L.X., M.L.B., X.L., C.L.H., J.Z., N.R., F.K., J.Q., D.Z., S.N., C.L., D.E.C., U.S., M.S.V., and K.E.H. are employees and stockholders of Pfizer Inc. J.R.A has received consultancy fees from Finch Therapeutics, Janssen, Pfizer Inc, Merck, and Takeda; has received grants from Merck; has served as a site Principal Investigator for Pfizer Inc for this trial. J.R. has no conflicts of interest to declare. E.J.S. has received grants from Abbott (AbbVie), AstraZeneca, Crohn’s and Colitis Foundation of America (CCFA), Celgene, Genentech, Janssen Research & Development, Johns Hopkins University, National Institute of Diabetes and Digestive and Kidney, National Institute of Health, New York Crohn’s Foundation, Pfizer Inc, Seres Therapeutics, UCB, and UCSF-CCFA Clinical Research Alliance; has received corporation consultant/advisory board fees from AbbVie, CCFA, Entera Health, Evidera, GI Health Foundation, Janssen, Protagonist Therapeutics, Seres Health, and Takeda Pharmaceuticals; holds stock in Gilead; has received honoraria from GI Health Foundation and Janssen for nonbranded speaker’s bureau. M.K. has received lecture fees from Alfa-Sigma, Ferring Pharmaceuticals, Janssen, Pharmabest, and Takeda; has received travel/accommodation/meeting expenses from Alfa-Sigma, Ferring Pharmaceuticals, Janssen, Pharmabest, Polish Foundation for Gastroenterology, and Takeda. S.D. has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ely Lilly, Enthera, Ferring Pharmaceuticals, Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer Inc, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB, and Vifor. R.L. has received consultancy fees from Janssen, Pfizer Inc, and Takeda.
Figures


References
-
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. - PubMed
-
- Siakavellas SI, Bamias G. Tumor necrosis factor-like cytokine TL1A and Its receptors DR3 and DcR3: important new factors in mucosal homeostasis and inflammation. Inflamm Bowel Dis. 2015;21:2441–2452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

