APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy
- PMID: 34427860
- PMCID: PMC8382940
- DOI: 10.1007/s12072-021-10239-x
APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy
Erratum in
-
Correction to: APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy.Hepatol Int. 2022 Apr;16(2):486-487. doi: 10.1007/s12072-022-10301-2. Epub 2022 Jan 25. Hepatol Int. 2022. PMID: 35076895 Free PMC article. No abstract available.
Abstract
Background & aim: Hepatitis B reactivation related to the use of immunosuppressive therapy remains a major cause of liver-related morbidity and mortality in hepatitis B endemic Asia-Pacific region. This clinical practice guidelines aim to assist clinicians in all disciplines involved in the use of immunosuppressive therapy to effectively prevent and manage hepatitis B reactivation.
Methods: All publications related to hepatitis B reactivation with the use of immunosuppressive therapy since 1975 were reviewed. Advice from key opinion leaders in member countries/administrative regions of Asian-Pacific Association for the study of the liver was collected and synchronized. Immunosuppressive therapy was risk-stratified according to its reported rate of hepatitis B reactivation.
Recommendations: We recommend the necessity to screen all patients for hepatitis B prior to the initiation of immunosuppressive therapy and to administer pre-emptive nucleos(t)ide analogues to those patients with a substantial risk of hepatitis and acute-on-chronic liver failure due to hepatitis B reactivation.
Keywords: APASL; Guideline; Hepatitis B reactivation; Immunosuppressive therapy.
© 2021. Asian Pacific Association for the Study of the Liver.
Conflict of interest statement
Ming-Lung Yu received research grants from Abbott, BMS, Gilead and Merck and received fees for being a speaker/consultant from Abbvie, Abbott, BMS, Gilead, Merck, Ipsen and Roche. Grace Wong has served as an advisory committee member for Gilead Sciences and Janssen, as a speaker for Abbott, Abbvie, Bristol-Myers Squibb, Echosens, Furui, Gilead Sciences, Janssen and Roche, and received research grant from Gilead Sciences. Alexander Thompson has served as an advisory committee member for Gilead Sciences, Abbvie, Roche, BMS, Merck, Immunocore, Janssen, Assembly Biosciences, Arbutus, Eisai, Ipsen and Bayer, as a speaker for Gilead Sciences, Abbvie, Roche, BMS, and received research grant from Gilead Sciences, Merck, BMS, Abbvie. Jin-Lin Hou received grants and personal fees from Bristol-Myers Squibb during the conduct of the study, and grants and personal fees from Bristol-Myers Squibb, GlaxoSmithKline, and Novartis. Teerha Piratvisuth received fees for being a speaker and advisory board member from Bayer, BMS, Gilead Sciences, and Eisai and received research support from Fibrogen, Gilead Sciences, Janssen and Roche. Ji-Dong Jia received consultation and speaker fees from Bristol-Myers Squibb, Gilead, Merck Sharp and Dohme, Novartis, and Roche. Ann-Lii Cheng received consultant fees from Novartis, Merck Serono, Eisai, Merck Sharp and Dohme, ONXEO, Bayer, Bristol-Myers Squibb, and Ono Pharmaceutical. Tony Mok received fees for being a speaker, consultant, and advisory board member from AstraZeneca, Roche/Genentech, Lilly, Bristol Myers Squibb, Boehringer Ingelheim, Novartis, Merck Sharp & Dohme, Pfizer, Merck Serono, SFJ Pharmaceuticals Group, ACEA Biosciences, Vertex, Celgene, Ignyta, Fishawack Facilitate Ltd, Takeda, Janssen, Hutchison MediPharma, and received grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, SFJ Pharmaceuticals Group, Roche, Merck Sharp & Dohme, Clovis Oncology, Bristol Myers Squibb, Xcovery. Diana A. Payawal has served as an advisory committee member for Mylan Pharmaceutical, as a speaker for Gilead Sciences, Mylan Pharmaceuticals, Echosense, Getz and Abbott. Tawesak Tanwandee received grants from Bristol-Myers Squibb and Merck. Masao Omata received fees for being a speaker, consultant, and advisory board member from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Otsuka, Astellas, Gilead Sciences, Chugai, Mitsubishi Tanabe, Kyorin, Merck Sharp and Dohme, Dainippon Sumitomo, Vertex Pharmaceuticals, Takeda, Merck Serono, and Zeria. George Lau, Hasmik Ghazinian, Masashi Mizokami, Gregory Cheng, Guo-Feng Chen, Zhen-Wen Liu, Oidov Baatarkhuu, Woon Leung Ng, Patrick Lau, Jer-Ming Chang, Saeed Hamid, A. Kadir Dokmeci, Rino A Gani, Diana A. Payawal, Pierce Chow, Joong-Won Park, Simone I Strasser, Rosmawaiti Mohamed, Khin Maung Win, Shiv Kumar Sari declare that they have no conflict of interest.
Figures
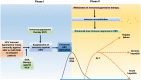
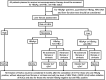
Comment in
-
Immune checkpoint inhibitors in chronic hepatitis B: therapy or high-risk factor for reactivation?Hepatol Int. 2022 Apr;16(2):480-481. doi: 10.1007/s12072-022-10317-8. Epub 2022 Mar 24. Hepatol Int. 2022. PMID: 35332508 No abstract available.
References
-
- Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68(1):105–112. - PubMed
-
- Hoofnagle JH, Dusheiko GM, Schafer DF, Jones EA, Micetich KC, Young RC, Costa J. Reactivation of chronic hepatitis B virus infection by cancer chemotherapy. Ann Intern Med. 1982;96(4):447–449. - PubMed
-
- Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100(1):182–188. - PubMed
-
- Lau G, Liang R, Chiu EK, Lee CK, Lam SK. Hepatic events after bone marrow transplantation in patients with hepatitis B infection: a case controlled study. Bone Marrow Transplant. 1997;19(8):795–799. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

