Long-Term Effectiveness of Natalizumab in Patients with Relapsing-Remitting Multiple Sclerosis Treated in the Routine Care in Greece: Results from the Multicenter, Observational 5-Year Prospective Study 'TOPICS Greece'
- PMID: 34427893
- PMCID: PMC8481212
- DOI: 10.1007/s40261-021-01073-y
Long-Term Effectiveness of Natalizumab in Patients with Relapsing-Remitting Multiple Sclerosis Treated in the Routine Care in Greece: Results from the Multicenter, Observational 5-Year Prospective Study 'TOPICS Greece'
Abstract
Background and objectives: For chronic diseases like multiple sclerosis (MS), real-world evidence on long-term treatment outcomes is essential. The study aimed to provide long-term data on the safety and effectiveness of natalizumab in patients with relapsing-remitting MS (RRMS) treated in a routine care setting in Greece.
Methods: TOPICS Greece was a multicenter, single-country, prospective 5-year observational study.
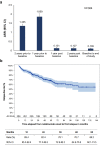
Results: Between 19-Apr-2012 and 18-Dec-2014, 304 eligible adults [females: 63.2%; median age at natalizumab initiation: 38.0 years; median disease duration: 6.2 years; median Expanded Disability Status Scale (EDSS) score at baseline: 3.5] were enrolled in the study by 20 hospital-based neurologists. The 1-year annualized relapse rate (ARR) before treatment initiation was 1.859, while the ARR during the first year of treatment was 0.131, representing a significant 93% reduction (p < 0.001). The ARR over the median treatment period of 59.4 months was 0.109. Patients with ≤1 relapse in the pre-natalizumab year (46.1%) and those having received ≤1 prior disease-modifying therapy (57.9%) displayed significantly lower on-natalizumab ARR. The 1-, 2-, 3-, 4- and 5-year cumulative probabilities of EDSS progression were 3.2, 6.2, 9.7, 13.4, and 17.4%, respectively; the respective probabilities of EDSS disability improvement were 18.3, 25.1, 27.4, 28.0, and 30.1%. Over a median safety data collection period of 48.7 months, 4.6% of the patients experienced ≥ 1 serious adverse event, with infections (reported in 1.0%) being the most common.
Conclusion: In real-world settings in Greece, natalizumab displayed beneficial long-term effects on disease activity and disability progression consistent with previous studies with no new serious safety signals emerging.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Prof. Dardiotis received grants, consulting fees and travel support from Bayer, Novartis, Genesis Pharma, Genzyme-Sanofi, Merck-Serono, Roche and Teva. Dr. Evangelopoulou received grants and consulting fees from Roche, Merck, Biogen, Teva and Genzyme. George Karachalios, Alexopoulou Tania, Rania Gourgioti are employees of Genesis Pharma SA. No other potential conflict of interest relevant to this article was reported.
Figures

References
-
- Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, Schwaninger M, Engelhardt B, de Vries HE. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 2016;1862(3):461–471. doi: 10.1016/j.bbadis.2015.10.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

