Phase II Study of Gemcitabine and Split-Dose Cisplatin Plus Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Bladder Cancer
- PMID: 34428076
- PMCID: PMC8478388
- DOI: 10.1200/JCO.21.01003
Phase II Study of Gemcitabine and Split-Dose Cisplatin Plus Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Bladder Cancer
Abstract
Purpose: To evaluate the safety and efficacy of gemcitabine and cisplatin in combination with the immune checkpoint inhibitor pembrolizumab as neoadjuvant therapy before radical cystectomy (RC) in muscle-invasive bladder cancer.
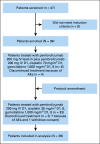
Methods: Patients with clinical T2-4aN0/XM0 muscle-invasive bladder cancer eligible for RC were enrolled. The initial six patients received lead-in pembrolizumab 200 mg once 2 weeks prior to pembrolizumab 200 mg once on day 1, cisplatin 70 mg/m2 once on day 1, and gemcitabine 1,000 mg/m2 once on days 1 and 8 every 21 days for four cycles. This schedule was discontinued for toxicity and subsequent patients received cisplatin 35 mg/m2 once on days 1 and 8 without lead-in pembrolizumab. The primary end point was pathologic downstaging (< pT2N0) with null and alternative hypothesis rates of 35% and 55%, respectively. Secondary end points were toxicity including patient-reported outcomes, complete pathologic response (pT0N0), event-free survival, and overall survival. Association of pathologic downstaging with programmed cell death ligand 1 staining was explored.
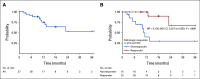
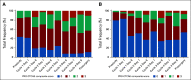
Results: Thirty-nine patients were enrolled between June 2016 and March 2020 (72% cT2, 23% cT3, and 5% cT4a). Patients received a median of four cycles of therapy. All patients underwent RC except one who declined. Twenty-two of 39 patients (56% [95% CI, 40 to 72]) achieved < pT2N0 and 14 of 39 (36% [95% CI, 21 to 53]) achieved pT0N0. Most common adverse events (AEs) of any grade were thrombocytopenia (74%), anemia (69%), neutropenia (67%), and hypomagnesemia (67%). One patient had new-onset type 1 diabetes mellitus with ketoacidosis related to pembrolizumab and no patients required steroids for immune-related AEs. Clinicians consistently under-reported AEs when compared with patients.
Conclusion: Neoadjuvant gemcitabine and cisplatin plus pembrolizumab met its primary end point for improved pathologic downstaging and was generally safe. A global study of perioperative chemotherapy plus pembrolizumab or placebo is ongoing.
Trial registration: ClinicalTrials.gov NCT02690558.
Conflict of interest statement
Figures


Comment in
-
Urological Oncology: Bladder, Penis and Urethral Cancer, and Basic Principles of Oncology.J Urol. 2022 Nov;208(5):1158-1159. doi: 10.1097/JU.0000000000002914. Epub 2022 Aug 19. J Urol. 2022. PMID: 35984094 No abstract available.
References
-
- International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al. : International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol 29:2171-2177, 2011 - PMC - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859-866, 2003 - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068-3077, 2000 - PubMed
-
- Powles T, Park SH, Voog E, et al. : Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218-1230, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

