Torquetenovirus in saliva: A potential biomarker for SARS-CoV-2 infection?
- PMID: 34428230
- PMCID: PMC8384193
- DOI: 10.1371/journal.pone.0256357
Torquetenovirus in saliva: A potential biomarker for SARS-CoV-2 infection?
Abstract
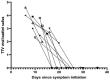
Torquetenovirus (TTV) is present in biological fluids from healthy individuals and measurement of its titer is used to assess immune status in individuals with chronic infections and after transplants. We assessed if the titer of TTV in saliva varied with the presence of SARS-CoV-2 in the nasopharynx and could be a marker of COVID-19 status. Saliva from 91 individuals positive for SARS-CoV-2 in nasal-oropharyngeal samples, and from 126 individuals who were SARS-CoV-2-negative, all with mild respiratory symptoms, were analyzed. Both groups were similar in age, gender, symptom duration and time after symptom initiation when saliva was collected. Titers of TTV and SARS-CoV-2 were assessed by gene amplification. Loss of smell (p = 0.0001) and fever (p = 0.0186) were more prevalent in SARS-CoV-2-positive individuals, while sore throat (p = 0.0001), fatigue (p = 0.0037) and diarrhea (p = 0.0475) were more frequent in the SARS-CoV-2 negative group. The saliva TTV and nasal-oropharyngeal SARS-CoV-2 titers were correlated (p = 0.0085). The TTV level decreased as symptoms resolved in the SARS-CoV-2 infected group (p = 0.0285) but remained unchanged in the SARS-CoV-2 negative controls. In SARS-CoV-2 positive subjects who provided 2-4 saliva samples and in which TTV was initially present, the TTV titer always decreased over time as symptoms resolved. We propose that sequential TTV measurement in saliva is potentially useful to assess the likelihood of symptom resolution in SARS-CoV-2-positive individuals and to predict prognosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Biagini P. Classification of TTV and related viruses (anelloviruses). Curr Top Microbial Immunol 2009;331:21–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

