MiRNA-1202 promotes the TGF-β1-induced proliferation, differentiation and collagen production of cardiac fibroblasts by targeting nNOS
- PMID: 34428251
- PMCID: PMC8384215
- DOI: 10.1371/journal.pone.0256066
MiRNA-1202 promotes the TGF-β1-induced proliferation, differentiation and collagen production of cardiac fibroblasts by targeting nNOS
Abstract
Background: Atrial fibrillation (AF) is a clinically common arrhythmia that affects human health. Myocardial fibrosis serves as an important contributor to AF. Recently, miRNA-1202 have been reported to be up-regulated in AF. However, the role of miRNA-1202 and its mechanism in myocardial fibrosis remain unclear.
Methods: Human cardiac fibroblasts (HCFs) were used to construct a fibrosis model by TGF-β1 induction. The expression of miR-1202 was measured by qRT-PCR. Cell proliferation was assessed by CCK-8 assays. Protein expression levels were measured by western blot. Collagen accumulation was measured by ELISA. The relationship between miR-1202 and nNOS was investigated by luciferase reporter assays.
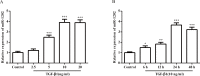
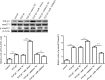
Results: MiR-1202 expression was obviously increased in HCFs and was both time- and dose-independent. MiR-1202 could increase the proliferation and collagen I, collagen III, and α-SMA levels with or without TGF-β1. MiR-1202 could also increase TGF-β1 and p-Smad2/3 protein levels in comparison to the control group. However, they were obviously decreased after inhibitor transfection. MiR-1202 targets nNOS for negative regulation of HCFs fibrosis by decreasing cell differentiation, collagen deposition and the activity of the TGF-β1/Smad2/3 pathway. Co-transfection of miR-1202 inhibitor and siRNA of nNOS inhibited nNOS protein expression, thereby enhancing the HCFs proliferation. Furthermore, co-transfection of the miR-1202 inhibitor and siRNA of nNOS significantly promoted collagen I, collagen III, TGF-β1, Smad2/3 and α-SMA protein expression and Smad2/3 protein phosphorylation. These findings suggested that miR-1202 promotes HCFs transformation to a pro-fibrotic phenotype by targeting nNOS through activating the TGF-β1/Smad2/3 pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
-
- Xing XQ XU J. Myocardial Fibrosis Related to Occurrence and Maintenance of Atrial Fibrillation. Advances in Cardiovascular Diseases. 2009; 30(1):164–167.
-
- Brilla CG, Reams GP, Maisch B, Weber KT. Renin–angiotensin system and myocardial fibrosis in hypertension: regulation of the myocardial collagen matrix. Eur Heart J. 1993; 14Suppl J:57–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

