Comparison of analytical sensitivity of SARS-CoV-2 molecular detection kits
- PMID: 34428543
- PMCID: PMC8379823
- DOI: 10.1016/j.ijid.2021.08.043
Comparison of analytical sensitivity of SARS-CoV-2 molecular detection kits
Abstract
Objectives: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has had a significant impact on global public health systems, making nucleic acid detection an important tool in epidemic prevention and control. Detection kits based on real-time reverse transcriptase PCR (rRT-PCR) have been used widely in clinics, but their analytical sensitivity (limit of detection, LOD) remains controversial. Moreover, there is limited research evaluating the analytical sensitivity of other molecular detection kits.
Methods: In this study, armored ribonucleic acid reference materials developed in-house were used to evaluate the analytical sensitivity of SARS-CoV-2 detection kits approved by the National Medical Products Administration. These were based on rRT-PCR and other molecular detection assays.
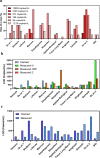
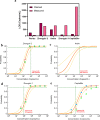
Results: The percentage retesting required with rRT-PCR kits was as follows: 0%, 7.69%, 15.38%, and 23.08% for samples with concentrations ranging from 50 000 to 781 copies/ml. In total, 93% of rRT-PCR kits had a LOD <1000 copies/ml. Only one kit had an LOD >1000 copies/ml. The LOD of other molecular detection kits ranged from 68 to 2264 copies/ml.
Conclusions: The study findings can help pharmaceutical companies optimize and improve detection kits, guide laboratories in selecting kits, and assist medical workers in their daily work.
Keywords: Analytical sensitivity; Limits of detection; Molecular detection; SARS-CoV-2; rRT-PCR.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

