SCD1, autophagy and cancer: implications for therapy
- PMID: 34429143
- PMCID: PMC8383407
- DOI: 10.1186/s13046-021-02067-6
SCD1, autophagy and cancer: implications for therapy
Abstract
Background: Autophagy is an intracellular degradation system that removes unnecessary or dysfunctional components and recycles them for other cellular functions. Over the years, a mutual regulation between lipid metabolism and autophagy has been uncovered.
Methods: This is a narrative review discussing the connection between SCD1 and the autophagic process, along with the modality through which this crosstalk can be exploited for therapeutic purposes.
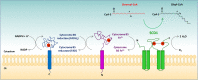
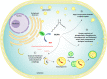
Results: Fatty acids, depending on the species, can have either activating or inhibitory roles on autophagy. In turn, autophagy regulates the mobilization of fat from cellular deposits, such as lipid droplets, and removes unnecessary lipids to prevent cellular lipotoxicity. This review describes the regulation of autophagy by lipid metabolism in cancer cells, focusing on the role of stearoyl-CoA desaturase 1 (SCD1), the key enzyme involved in the synthesis of monounsaturated fatty acids. SCD1 plays an important role in cancer, promoting cell proliferation and metastasis. The role of autophagy in cancer is more complex since it can act either by protecting against the onset of cancer or by promoting tumor growth. Mounting evidence indicates that autophagy and lipid metabolism are tightly interconnected.
Conclusion: Here, we discuss controversial findings of SCD1 as an autophagy inducer or inhibitor in cancer, highlighting how these activities may result in cancer promotion or inhibition depending upon the degree of cancer heterogeneity and plasticity.
Keywords: Autophagy; Lipid metabolism; cancer.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Cahová M, Daňková H, Páleníčková E, Papáčková Z, Kazdová L. The autophagy-lysosomal pathway is involved in TAG degradation in the liver: the effect of high-sucrose and high-fat diet. Folia Biol. 2010;56(4):173–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

