Human papillomavirus (HPV) vaccine introduction in Sikkim state: Best practices from the first statewide multiple-age cohort HPV vaccine introduction in India-2018-2019
- PMID: 34429233
- PMCID: PMC10874777
- DOI: 10.1016/j.vaccine.2021.07.024
Human papillomavirus (HPV) vaccine introduction in Sikkim state: Best practices from the first statewide multiple-age cohort HPV vaccine introduction in India-2018-2019
Abstract
Background: Cervical cancer is a leading cause of cancer-associated mortality among women in India, with 96,922 new cases and 60,078 deaths each year, almost one-fifth of the global burden. In 2018, Sikkim state in India introduced human papillomavirus (HPV) vaccine for 9-13-year-old girls, primarily through school-based vaccination, targeting approximately 25,000 girls. We documented the program's decision-making and implementation processes.
Methods: We conducted a post-introduction evaluation in 2019, concurrent with the second dose campaign, by interviewing key stakeholders (state, district, and local level), reviewing planning documents, and observing cold chain sites in two purposefully-sampled community areas in each of the four districts of Sikkim. Using standard questionnaires, we interviewed health and education officials, school personnel, health workers, community leaders, and age-eligible girls on program decision-making, planning, training, vaccine delivery, logistics, and communication.
Results: We conducted 279 interviews and 29 observations in eight community areas across four districts of Sikkim. Based on reported administrative data, Sikkim achieved >95% HPV vaccination coverage among targeted girls for both doses via two campaigns; no severe adverse events were reported. HPV vaccination was well accepted by all stakeholders; minimal refusal was reported. Factors identified for successful vaccine introduction included strong political commitment, statewide mandatory school enrollment, collaboration between health and education departments at all levels, and robust social mobilization strategies.
Conclusions: Sikkim successfully introduced the HPV vaccine to multiple-age cohorts of girls via school-based vaccination, demonstrating a model that could be replicated in other regions in India or similar low- and middle-income country settings.
Keywords: Cervical cancer; HPV vaccine; School-based vaccination; Sikkim; Vaccine introduction.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


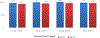
Similar articles
-
National introduction of human papillomavirus (HPV) vaccine in Tanzania: Programmatic decision-making and implementation.Vaccine. 2022 Mar 31;40 Suppl 1(Suppl 1):A2-A9. doi: 10.1016/j.vaccine.2021.04.025. Epub 2021 May 4. Vaccine. 2022. PMID: 33962839 Free PMC article. Review.
-
Tanzania's human papillomavirus (HPV) vaccination program: Community awareness, feasibility, and acceptability of a national HPV vaccination program, 2019.Vaccine. 2022 Mar 31;40 Suppl 1(Suppl 1):A38-A48. doi: 10.1016/j.vaccine.2021.06.047. Epub 2021 Jul 3. Vaccine. 2022. PMID: 34229889 Free PMC article.
-
Community-based household assessment of human papillomavirus (HPV) vaccination coverage and acceptability - HPV vaccine demonstration program, Cambodia - 2017.Vaccine. 2019 Feb 21;37(9):1202-1208. doi: 10.1016/j.vaccine.2018.12.052. Epub 2019 Jan 24. Vaccine. 2019. PMID: 30686637 Free PMC article.
-
Challenges and lessons from a school-based human papillomavirus (HPV) vaccination program for adolescent girls in a rural Nigerian community.BMC Public Health. 2022 Aug 24;22(1):1611. doi: 10.1186/s12889-022-13975-3. BMC Public Health. 2022. PMID: 36002832 Free PMC article.
-
Current status of human papillomavirus vaccination in India's cervical cancer prevention efforts.Lancet Oncol. 2019 Nov;20(11):e637-e644. doi: 10.1016/S1470-2045(19)30531-5. Lancet Oncol. 2019. PMID: 31674322 Review.
Cited by
-
Digitizing tools for post introduction evaluation of rotavirus vaccine introduction in India.Vaccine X. 2024 May 23;19:100502. doi: 10.1016/j.jvacx.2024.100502. eCollection 2024 Aug. Vaccine X. 2024. PMID: 38827494 Free PMC article.
-
Effect of health education on acceptance of human papilloma virus vaccine among parents of adolescent girls of Bishnupur, Manipur: A quasi-experimental study.Indian J Med Res. 2024 Sep&Oct;160(3&4):346-353. doi: 10.25259/IJMR_49_2024. Indian J Med Res. 2024. PMID: 39632632 Free PMC article.
-
Understanding cervical cancer, human papillomavirus (HPV), and HPV vaccine acceptance in college-going students: Institutional-based cross-sectional study from Bihar State.J Family Med Prim Care. 2025 Jan;14(1):363-370. doi: 10.4103/jfmpc.jfmpc_1277_24. Epub 2025 Jan 13. J Family Med Prim Care. 2025. PMID: 39989516 Free PMC article.
-
HPV Vaccines - An Overview.Indian J Dermatol. 2025 Jul-Aug;70(4):188-200. doi: 10.4103/ijd.ijd_805_24. Epub 2025 Jun 30. Indian J Dermatol. 2025. PMID: 40740403 Free PMC article. Review.
-
Multilevel Targets for Promoting Pediatric HPV Vaccination: A Systematic Review of Parent-Centered, Provider-Centered, and Practice-Centered Interventions in HIC and LMIC Settings.Vaccines (Basel). 2025 Mar 11;13(3):300. doi: 10.3390/vaccines13030300. Vaccines (Basel). 2025. PMID: 40266195 Free PMC article. Review.
References
-
- Globocan: Global Cancer Observatory India Fact Sheet. In: International Agency for Research on Cancer WHO, editor. Geneva, Switzerland: WHO; 2018.
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. - PubMed
-
- WHO position on HPV vaccines. Vaccine 2009;27:7236–7. - PubMed
-
- Human papillomavirus vaccines. WHO position paper, May 2017-Recommendations. Vaccine 2017;35:5753–5. - PubMed

