Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function
- PMID: 34429382
- PMCID: PMC8613804
- DOI: 10.1126/scisignal.abe2606
Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function
Abstract
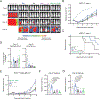
Chimeric antigen receptor (CAR)-modified T cell therapy is effective in treating lymphomas, leukemias, and multiple myeloma in which the tumor cells express high amounts of target antigen. However, achieving durable remission for these hematological malignancies and extending CAR T cell therapy to patients with solid tumors will require receptors that can recognize and eliminate tumor cells with a low density of target antigen. Although CARs were designed to mimic T cell receptor (TCR) signaling, TCRs are at least 100-fold more sensitive to antigen. To design a CAR with improved antigen sensitivity, we directly compared TCR and CAR signaling in primary human T cells. Global phosphoproteomic analysis revealed that key T cell signaling proteins-such as CD3δ, CD3ε, and CD3γ, which comprise a portion of the T cell co-receptor, as well as the TCR adaptor protein LAT-were either not phosphorylated or were only weakly phosphorylated by CAR stimulation. Modifying a commonplace 4-1BB/CD3ζ CAR sequence to better engage CD3ε and LAT using embedded CD3ε or GRB2 domains resulted in enhanced T cell activation in vitro in settings of a low density of antigen, and improved efficacy in in vivo models of lymphoma, leukemia, and breast cancer. These CARs represent examples of alterations in receptor design that were guided by in-depth interrogation of T cell signaling.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
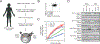




References
-
- Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG, CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients, J. Clin. Invest 126, 2123–2138 (2016). - PMC - PubMed
-
- Turtle CJ, Hanafi L-A, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, Heimfeld S, Riddell SR, Maloney DG, Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells, Sci Transl Med 8, 355ra116 (2016). - PMC - PubMed
-
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY, Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma, N Engl J Med 377, 2531–2544 (2017). - PMC - PubMed
-
- Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Spratt KS, Hoglund V, Lindgren C, Oron AP, Li D, Riddell SR, Park JR, Jensen MC, Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults, Blood 129, 3322–3331 (2017). - PMC - PubMed
-
- Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA, Kammula US, Sherman M, Perez A, Yuan CM, Feldman T, Friedberg JW, Roschewski MJ, Feldman SA, McIntyre L, Toomey MA, Rosenberg SA, Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels, J. Clin. Oncol, JCO2016713024 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

